Brittle-Ductile Transition Temperature
(Shear-Yielding and Crazing)
Polymeric materials under stress can undergo plastic deformation before breaking or they can fracture without appreciable plastic flow.1 Brittle failure is characterized by low strain and fracture at the highest stress whereas ductile fracture involves a large degree of plastic deformation and fracture not necessarily occurs at the highest stress.
Because of their viscoelastic properties, the fracture behavior of polymeric materials varries considerably with temperature. Below the brittle-ductile transition temperature, polymers fail via crazing or shear yielding wheras above this temperature yielding dominates. The brittle-ductile transition temperature itself strongly depends on the morphology and the chain structure of the polymer. Polymers with a flexible backbone tend to have a much lower brittle-ductile transition temperature than polymers with a stiff backbone. This behavior can be explained in terms of entanglements and free volume.
Entanglements behave like physical cross-links and increase the resistance to void formation/growth and crack propagation. High molecular weight polymers with a low entanglement density and a large characteristic ratio tend to craze whereas polymers with high entanglement density and small characteristic ratio tend to yield. Thus, with decreasing entanglement density, the brittle-ductile transition shifts to higher temperatures.
The brittle-ductile transition temperature of a polymer also depend on its molecular weight (MW). Lowering the molecular weight greatly increases the mobility of the polymer chains. Thus, a lower MW polymer has a greater tendency to yield than a higher MW polymer, i.e. with decreasing MW, the brittle-ductile transition shifts to lower temperatures.
The prevailing view is that materials below their glass transition are brittle. However, this is only the case for polymers with large bulky side groups such as polystyrene or polyphenyl methacrylate. In this case, the the brittle-ductile transition temperature coincides with the α-transtion temperature,
Tb = Tα = Tg
For the majority of polymers the ductile-brittle transition occurs at a much lower temperature. For most amorphous polymers, vitrification or glassification coincides with the β-transtion temperature,
Tb = Tβ
and for very tough polymers such as polycarbonate and polysulfone and many semic-crystalline polymers the brittle-ductile transition corresponds with the γ-transtion temperature,
Tb = Tγ
A number of relationships have been proposed to relate the β-transition temperature to the glass transition temperature. For example, Boyer2 suggested Tb / Tg ≈ 0.75. However, no such simple relationships exist. As has been shown by Wu, the brittle-to-ductile transition temperature is a function of the intrinsic chain stiffness or flexibility. Wu3,4 found following relationship:
{Tβ} / {Tα} = Tb / Tg = 0.135 + 0.082 C∞
which is only applicable to polymers with Tb < Tg , i.e. polymers without bulky side groups. This is the case for polymers with C∞ ≤ 10.5.
Brittle-Ductile Transition of some Polymers,
TB / TG versus C∞ (1 Hz)
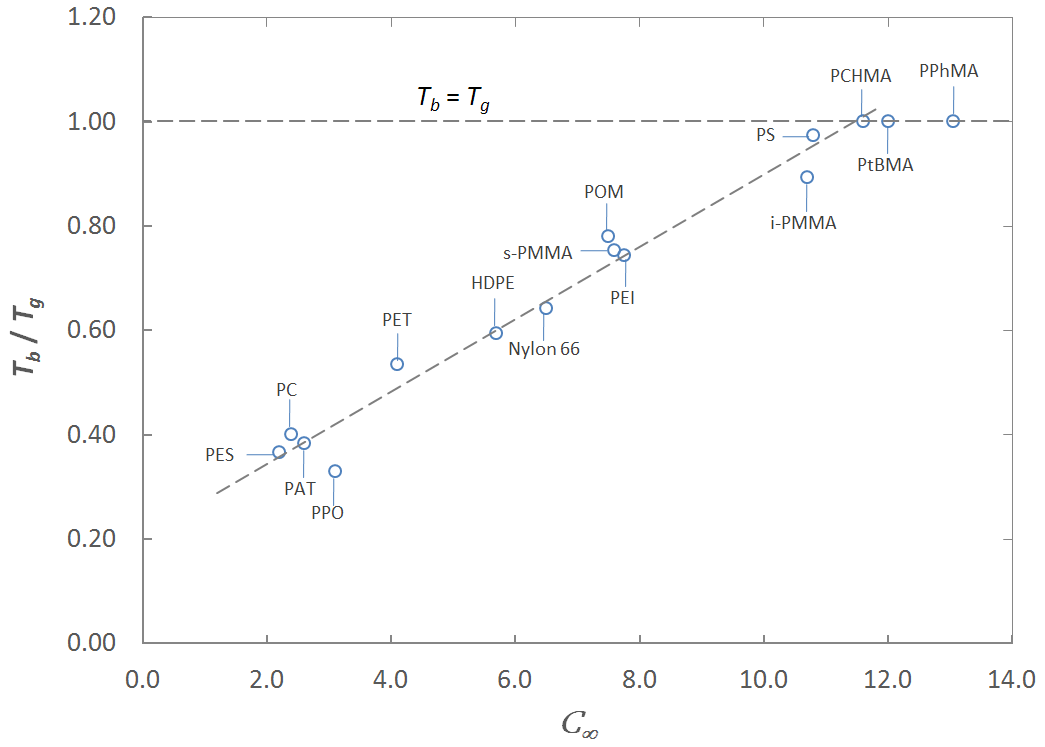
With the Tb - Tg data from several sources for both amorhpous and semi-crystalline polymers we find a similar relationship:
Tb / Tg = 0.208 + 0.069 C∞
Thus, the ratio Tb / Tg is indeed a function of the chain stiffness (C∞) (see Figure above).
References & Notes
The ability of a solid material to undergo significant plastic deformation before rupture is called ductility, which is typically expressed as percent elongation at break.
R. F. Boyer, Polymer 17, 996 (1976).
Souheng Wu, J. Appl. Poly. Sci., Vol. 46, 619 - 624 (1992)
Souheng Wu, Polymer lnternational, 29, 229 - 247 (1992)