Flow Properties of Polymers
Time-independent Fluids
Polymer solutions, dispersions, and melts are usually non-Newtonian liquids. This means their apparent viscosity (η)1 depends on the applied shear rate and increases rapidly with increasing molecular weight (number of repeat units). Thus, the viscosity of a polymer melt is always larger than that of the corresponding monomer. This is due to entanglement and intermolecular forces between polymer molecules.
The shear rate (γ) - shear stress (τ) relationship of time independent non-Newtonian fluids can be described by the general equation
![]()
or graphically by a curve of shear stress as a function of shear rate. The four basic types of time-independent fluids are shown in the figures below.
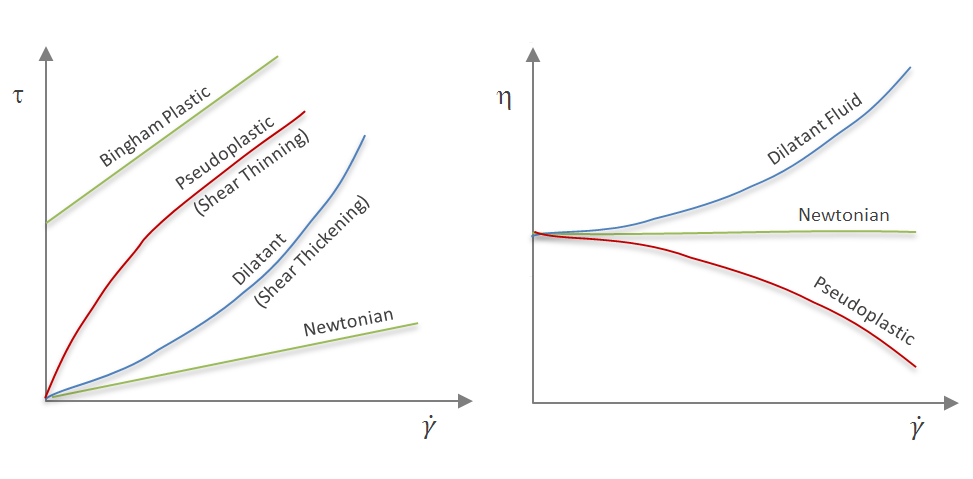
It must be emphazised that these types are an idealization of the real flow behavior of fluids. Most polymer solutions and melts exhibit shear thinning, that is, they belong to the class of pseudoplastic materials, whereas shear-thickening or dilatant behavior is rarely observed. Some common examples of shear-thickening fluids are cornstarch in water and nanoparticles dispersed in a (polymer) solution.
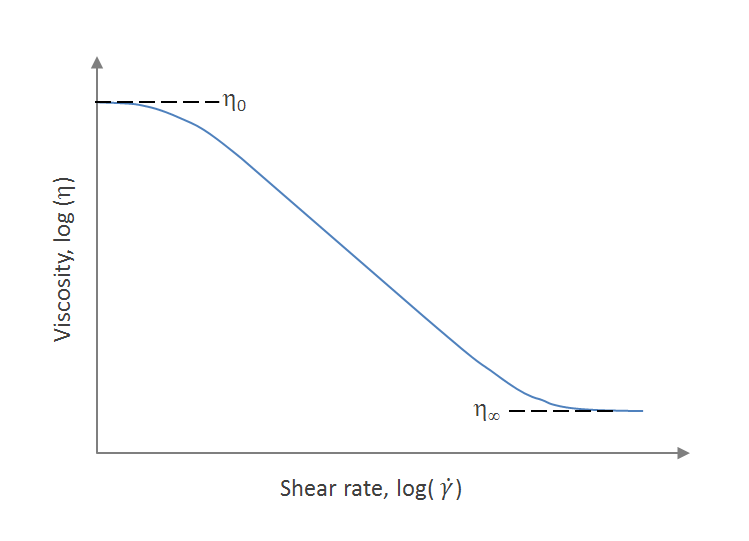
The observed shear thinning of polymer melts and solutions is caused by disentanglement of polymer chains during flow. Polymers with a sufficiently high molecular weight are always entangled (like spagetti) and randomly oriented when at rest. When sheared, however, they begin to disentangle and to allign which causes the viscosity to drop. The degree of disentanglement will depend on the shear rate. At sufficiently high shear rates the polymers will be completely disentangled and fully alligned. In this regime, the viscosity of the polymer melt or solution will be independent of the shear rate, i.e. the polymer will behave like a Newtonian liquid again.2 The same is true for very low shear rates; the polymer chains move so slowly that entanglement does not impede the shear flow. The viscosity at infinite slow shear is called zero shear rate viscosity (η0). The typical behavior is ilustrated in the figure below that shows the dependence of the apparent viscosity, η, of a polymeric melt on shear rate.
The behavior of fluids in the shear-thinning regime can be described with the power-law equation of Oswald and de Waele:
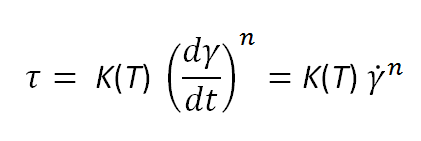
This equation may be written in logarithmic form,
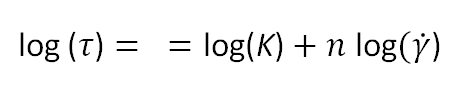
This means, a log-log plot of shear stress (τ) versus shear strain (dγ/dt) should yield a straight line if the polymer solution or melt behaves like a pseudoplastic liquid. Usually a straight line can be drawn over one to two decades of shear rate, but over a wider range deviations from the Oswald law can be expected.
The apparent viscosity is defined by
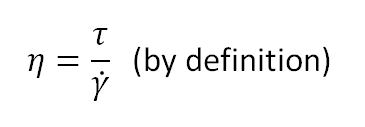
If we combine this expression with the Oswald equation, we obtain a second power-law equation for the apparent viscosity:
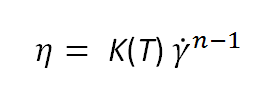
A power law can also be used to describe the behavior of a dilatant (shear-thickening) liquid. In this case, the value of the exponent n will be greater than one. Again, noticeable deviations can be expected when the Oswald equation is applied over a wider range of shear rates.
Some other fluids require a threshold shear stress before they start to flow. This kind of fluid is called a plastic fluid and if the flowing liquid has a constant viscosity it is called a Bingham liquid. However, such behavior is not observed in ordinary polymer melts and solutions. Typical examples for plastic flow behavior are polymer/silica micro- and nanocomposites. The solid-like behavior at low shear stress can be explained by the formation of a silica network structure arising from attractive particle-particle interactions due to hydrogen bonding between silanol groups. Once the particle network breaks down upon application of a critical yield stress (τy), the polymer shows normal flow behavior.
The flow behavior of plastic fluids having a constant viscosity ηp above the yield stress can be described with the Bingham equation:
![]()
whereas non-Newtonian (shear-thinning) behavior of a plastic fluid can be described with the Herschel-Bulkley model:
![]()
Using the standard definition for viscosity: η = τ / γ, the apparent viscosity of an Bingham viscoplastic material can be written as
![]()
Thus, the apparent viscosity of a Bingham fluid decreases with increasing shear rate and reaches at very high shear rates the constant limit ηp.
1The apparent viscosity is often given the symbol η instead of μ to distinguish it from the Newtonian viscosity.
2The second plateau is rarley observed for polymer melts because it requires extremely high shear rates which might also cause the polymer chains to break (shear-induced degradation).