Stress Relaxation Processes
In a stress relaxation experiment, a sample is rapidly strained to a fixed length at constant temperature and the stress is recorded as a function of time. Since the length after stretching the sample is kept constant for the entire duration of the experiment, no macroscopic movement of the test specimen is possible. Therefore, stress relaxation can only occur on a molecular level, i.e. by molecular relaxation and viscous flow. Creep experiments are conducted in the inverse manner; instead of keeping the strain constant, a constant stress is applied and the creep (strain) is recorded as a function of time. Stress relaxation experiments are often reported as time-dependent modulus, E(t), whereas creep experiment are reported as time-dependent compliance J(t) = 1/E(t).
Several relaxation processes are possible that can be grouped in several categories. The most important relaxation mechanisms are viscous flow, molecular relaxation, disentanglement of physical cross-links (knots), bond interchange and chain scission.
Viscous flow is caused by (linear) polymer chains moving past one another. This relaxation mechanism becomes important when the temperature increases and the chains gain mobility, especially near or above the glass transition temperature. The same is true for relaxation through molecular motion. Relaxation is mainly caused by rotation of bonds, for example by a "crankshaft" mechanism. Near the Tg the chains relax at about the same rate as the time frame of the experiment. The motions can be grouped in main-chain and side-chain motions. In general, low-temperature main-chain motions absorb energy better than equivalent side-chain motions.
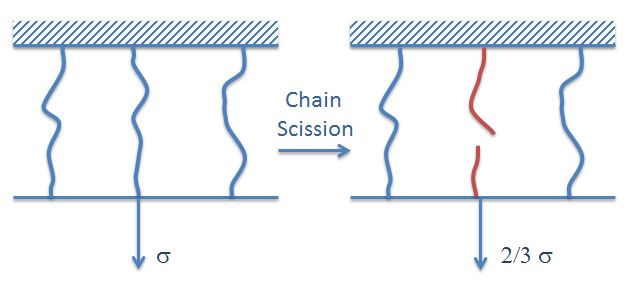
Chain scission is often caused by oxidation and hydrolysis and/or an applied stress at elevated temperature, that is, the reduction in stress is caused by breaks in load bearing chains. This relaxation is of little importance at low temperature, but gains importance with increasing temperature and exposure time and is the dominating relaxation mechanism when the temperature approaches the decomposition temperature.
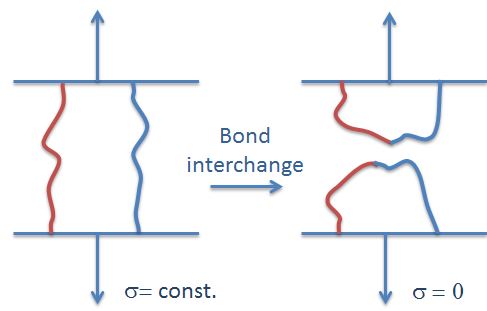
Bond interchange often occurs in polyesters and polysiloxanes. In this case, stress relaxation is achieved by interchange of bonds perpendicular to the stress vector, that is, the stress is relaxed in a similar manner as chain scission, only that two pairs of neighboring "broken" chain ends form two new (not load bearing) chains.
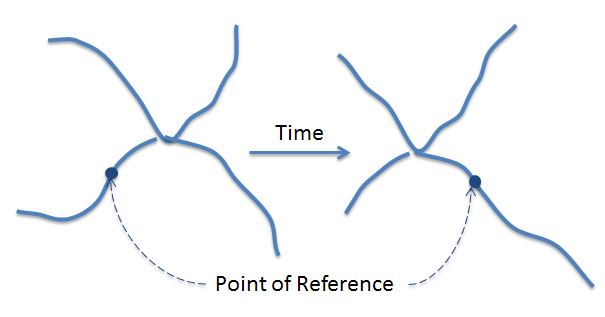
Disentanglement of physical knots and trapped entanglements in elastic networks is caused by chain diffusion and can be explained with the Rouse-Bueche or the de Gennes Reptation model, that describes polymer chain diffusion as a snake-like motion within a tube formed by "fixed" obstacles, i.e. by other chains and other particles (plasticizer molecules, etc.).