Second-Order Transitions
Variations of Properties at Glass Transition Temperature
The glass transition can be considered an Ehrenfest second-order transition. This means a discontinuity in the second derivative of the Gibbs free energy is observed when the temperature reaches the glass transition point. Both the volume and the entropy of a polymer will change abruptly. In contrast to real second-order transitions, the change of the thermodynamic properties (slope of the curve) is more gradual and is also affected by the heating rate. For this reason, the glass transition is only a quasi or pseudo second-order thermodynamic transition.
Besides volume and entropy, several other properties will change more or less abruptly when the glass transition temperature is reached. For example, discontiuities in the slope as a function of temperature are observed for the molar volume (v), thermal expansion coefficient (α), enthalpy (H), heat capacity (Cp), shear and tensile storage modulus (G, E), and refractive index (n) as shown schematically in the figures below.
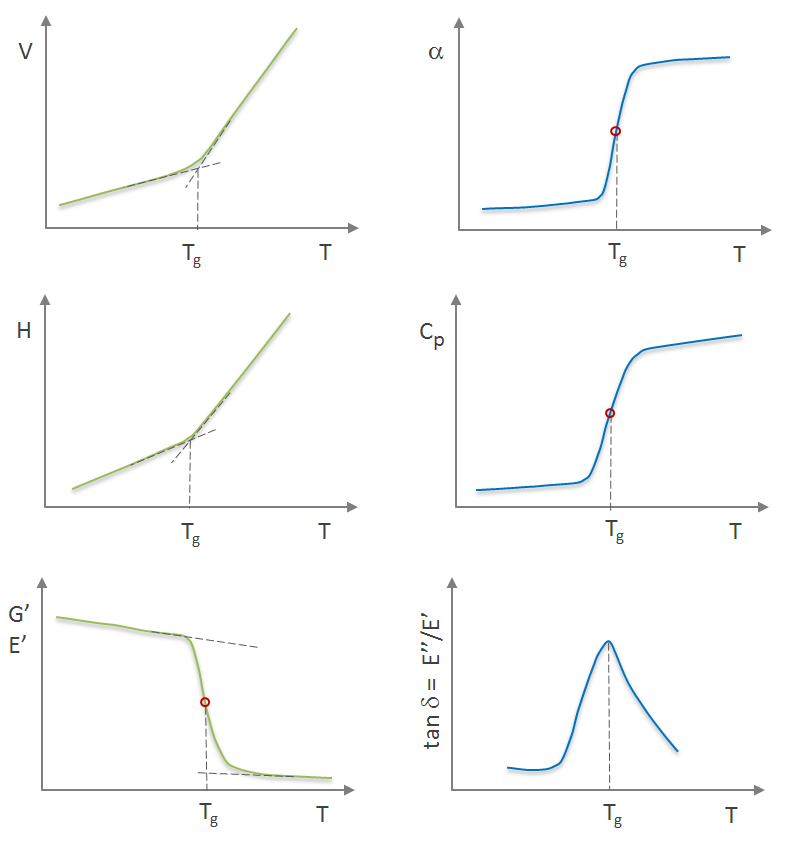
The viscoelastic, calometric, and volumetric properties and the glass transition temperature can be measured with various analytical methods. The most common methods are DMA, DSC, and TMA.