Entropy Continuity
and Discontinuity in Specific Heat at Tg
At the glass transition, we can assume a two-phase equilibrium, that is, the pure-component specific entropies in the liquid (rubbery) and glassy state should be identical:
Sglass(T,p) = Sliq(T,p)
and at constant pressure or temperature:
(∂H / ∂T)p = T(∂S / ∂T)p = Cp
(∂S / ∂P)T = -(∂Vm / ∂T)P = -Vm · α
where α is the coefficient of volumetric thermal expansion and Vm the molar volume. Inserting these two equations into the thermodynamic continuity statement
(∂Sglass / ∂T)pdT + (∂Sglass / ∂P)Tdp = (∂Sliq / ∂T)pdT + (∂Sliq / ∂P)Tdp
gives
Cp, glass d lnT - Vm,glass αglass dp = Cp,liq d lnT - Vm,liq αliq dp
or
(∂ lnTg / ∂P) = ( Vm,liq αliq - Vm,glass αglass) / ( Cp,liq - Cp,glass)
Discontinuity at Glass Transition
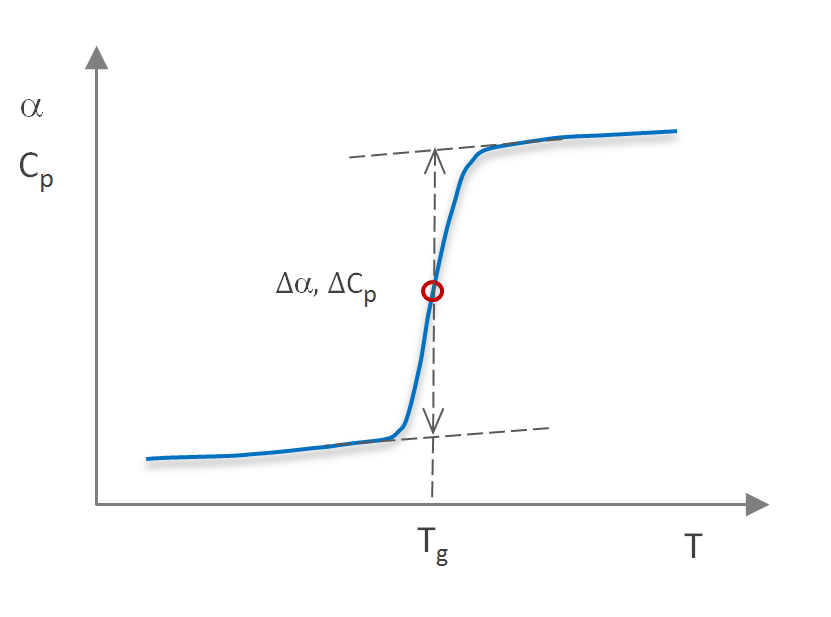
Since volume continuity at Tg requires that Vm,glass = Vm,liq, this expression reduces to
(∂ lnTg / ∂P) = Vm Δα / ΔCp
or
(∂ Tg / ∂P) = Vm Tg Δα / ΔCp
where ΔCp and Δα are the discontinuity in specific heat and coefficient of thermal expansion at the glass transition temperature. For most polymers, ΔCp is in the range of 0.2 - 0.3 J/(g-K).