Phase Behavior Prediction
(Ruzette-Mayes Model)
Part 2
The thermodynamics of (binary) polymer solutions and blends can be described with the (generalized) Flory-Huggins theory1-3. However, this theory has several short-commings; in the Flory-Huggins formalism, the binary interaction parameter, χi,j, is a complex function of several independent variables that are often unknown and usually have to be measured. The compressible regular solution free energy model of Ruzette and Mayes (2001)6,7, on the other hand, makes only use of pure component properties which are assumed to be concentration and temperature dependent, that is, this model avoids the direct calculation of the temperature and concentration dependency of the interaction parameter. The model is based on a modification of Flory and Huggins regular solution free energy model and captures, at least semi-quantitatively, the phase behavior of weakly interacting polymers in blends and solutions.
Pure Component Properties
The four pure component properties needed for the prediction of the phase curves (binodals and spinodals) are the mass density, the molar hard-core density, the solubility parameter, and the thermal expansion coefficient.
The molar hard-core volume of a repeat (vhc) is the ratio of the molecular weight of a repeat unit and the hard core density, vhc = Mr / ρhc. If vhc is unknown, it can be calculated from the van der Waals volume, vhc ≈ 1.25 vvw.4
The thermal expansion coefficient (α) has been measured for many common polymers. If unknown, α together with vhc can be obtained from P-V-T data. These data are available in the literature for many homopolymers over a certain temperature range. Both parameters can be computed by fitting these data from the melt-state regime to the following form:
ρ = ρhc exp(-αT)
In some cases, P-V-T data for the (hypothetical) melt state are not available; in that case, they can be predicted with the so-called Tait equation:
ρ(1bar,T) ≈ ρ(0,T) = ρ(p,T) {1 - C ln[1 + p / B(T)]}
The constants of the Tait equation have been calculated for many common polymers by Simha (1973), Prausnitz (1975) and several other researchers. C has an average value of about 0.0894. The other factor (B) is a function of temperature:
B(T) = b1 exp(-b2T)
Both b1 and b2 depend on the nature of the polymer. As has been shown by Simha and others (1973), the Tait equation is valid for polymers in the glassy state as well.
A similar approach can be used to determine the temperature dependent solubility parameters. The values of δ(298K) can be either measured or calculated with group contributions methods. These values can then be extrapolated to other temperatures:
δ(T) = δ(298K) · [ρhc(T) / ρhc(298K)]1/2
Phase Diagrams of Binary Blends
Once all pure component properties have been determined, the binodal and spinodal phase curves for weakly interacting polymer pairs can be easily calculated. The stability criterion for spinodal points5 is the second derivative of the intensive free energy of mixing with respect to composition:
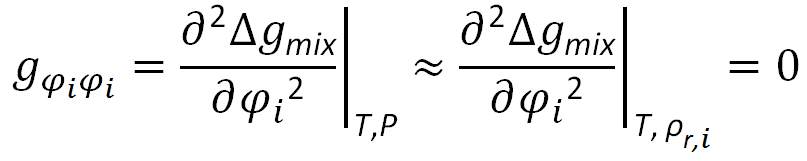
When applied to the Gibbs free energy of the Ruzette-Mayes model6,7, following mathematical expression for the stability condition can be derived:
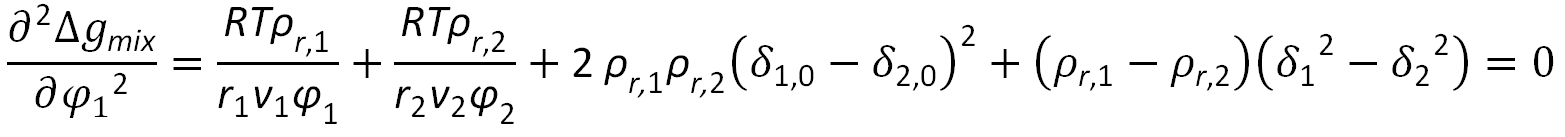
where ρr,i = ρi / ρhc,i = vi / vhc,i is the reduced density, δ0,i2 = ρr,i δi2 is the corresponding hardcore cohesive energy density, and φ1 and φ2 are the volume fractions of the two components,
φi = Ni ri vi / (N1 r1 v1 + N2 r2 v2)
The spinodal condition has two solutions:
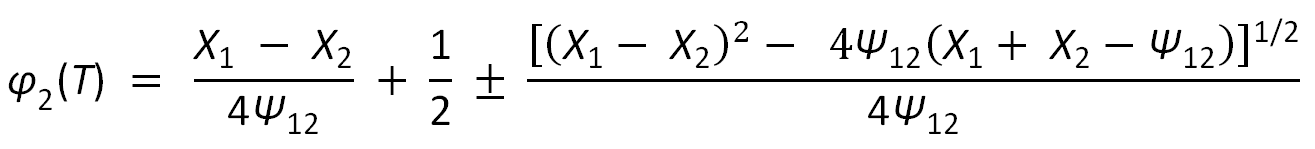
with
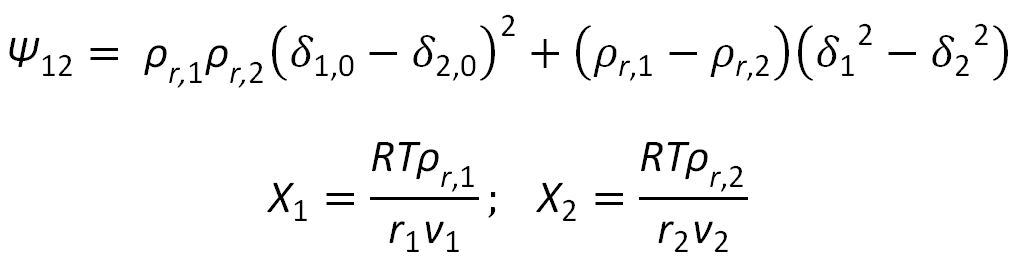
The classic equilibrium criteria for binodals reads
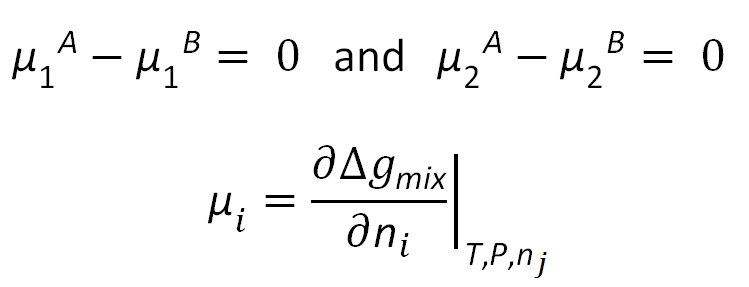
This condition states that the chemical potential of each component (solvent, polymer) should be equal in both phases. The chemical potentials of the components in each phase can be calculated using the following expression:
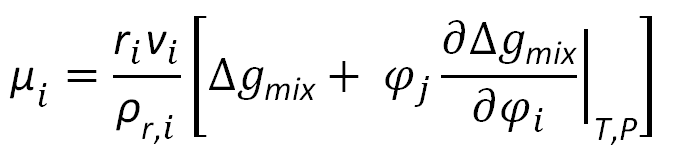
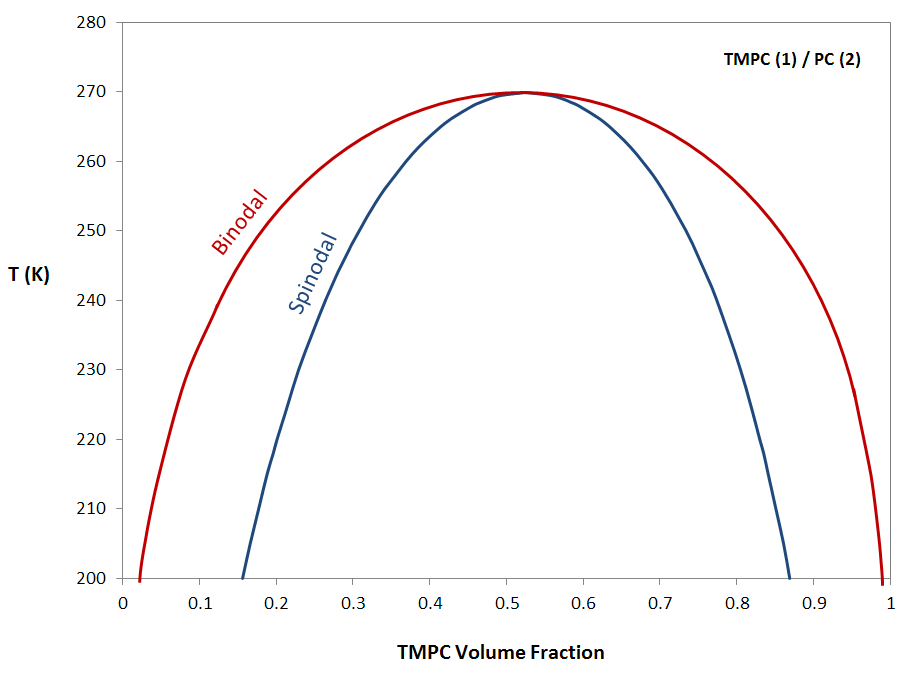
The compressible regular solution model presented here is only applicable to weakly interacting systems. An example for such a system is a blend of polycarbonate (PC) and polytetramethylcarbonate (TMPC). This system shows only an upper critical solution temperature as is shown in the figure below for MPTMC = 27 800 and MPC = 37 000 g/mol. This finding is consistent with the observed behavior for this mixture, that is, these polymers remain miscible up to their degradation temperature.7
Spinodal and Binodal of A TMPC/PC Blend
References and Notes
- P. J. Flory, J. Chem. Phys. 9, 660 (1941); 10, 51 (1942)
- M. L. Huggins, J. Phys. Chem. 46, 151 (1942); J. Am. Chem. Soc. 64, 1712 (1942)
- Paul L. Flory, Principles of Polymer Chemistry, Ithaca, New york, 1953
The hard core volume is the volume of a repeat unit at zero Kelvin and zero pressure. This volume is rather difficult to determine. It is often arbitrarily set equal to the van der Waals volume, vhc ≈ vvw. Others investigators used a different approach; today, most agree that vhc is equal to the closed packed volume of the molecules. For example, in the case of a closed-packed cubic structure, vhc ≈ 1.35 vvw
-
The points that separate the unstable region from the meta-stable region are called a spinodal points.
-
Anne-Valerie G. Ruzette and Anne M. Mayes, Macromolecules 34, 1894-1907 (2001)
-
Juan A. Gonzalez-Leon and Anne M. Mayes, Macromolecules 36, 2508-2515 (2003)