Cohesive Energy Density and Hildebrand Parameter
A quantitative measure for the cohesive properties of a polymer is the cohesive energy, Ecoh. The cohesive energy per unit volume is called cohesive energy density (ecoh) and the square root of the cohesive energy density the solubility or Hildebrand parameter, δh. This parameter is frequently used in the coating industry to aid in the selection of solvents and to predict compatibility of polymers. Solubility parameters are also used to predict the chemical resistance, the permeation rate, and the mechanical properties of polymers, to name only a few applications.
The cohesive energy (Ecoh) of a substance in a condensed state is defined as the increase in internal energy (U) per mole of substance if all intermolecular forces are eliminated. The correlations to the other two quantities are
ecoh (J/cm³ = MPa) = Ecoh / Vm
δh (MPa1/2) = (Ecoh / Vm)1/2= ecoh1/2
In the case of low molecular weight compounds, the cohesive energy, Ecoh, is the energy required to evaporate the material:
Ecoh ≈ ΔHvap - RT
whereas the cohesive energy of a polymer can only indirectly be measured because a high molecular compound usually decomposes before it evaporates. For this reason, cohesive energies are often calculated with group contribution methods.
The Hildebrand parameters of some common polymers are shown below. The values in the second column have been recommended by Barton and Wolfarth and the values in the third column have been calculated with a group contribution method.a
| Compound | Exper. δh (J/cm3)1/2 | Predicted δh (J/cm3)1/2 |
| Polyethylene (PE) | 16.8 | 16.5 |
| Polycarbonate (PC) | 20.0 | 20.3 |
| Poly(tetrahydrofuran) (PTMO) | 18.3 | 18.1 |
| Poly(caprolactone) (PLC) | 19.9 | 19.8 |
| Poly(ethylene sulfide) | 18.8 | 19.7 |
| Poly(ethylene terephthalate) (PET) | 21.9 | 22.0 |
The cohesive energy and the related parameters are of great importance in polymer physics, because many thermophysical and mechanical properties are directly related to the cohesive energy. For example, linear relationships were found between the cohesive energy, the heat capacity, and the shear modulus.2
The chart below depicts the glass transition temperature as a function of the normalized cohesive energy.3 In the absence of strong specific intermolecular interactions and side groups, the normalized cohesive energy is directly proportional to the glass transition temperature.
| Compound | Exper. Tg (K) | Ecoh / Nosca (KJ/mol) |
| Polyethylene (PE) | 193 | 4500 |
| Polycarbonate (PC) | 424 | 14400 |
| Poly(tetrahydrofuran) (PTMO) | 187 | 4740 |
| Poly(caprolactone) (PLC) | 307 | 5370 |
| Poly(glycolide) (PGL) | 208 | 10400 |
| Poly(ethylene sulfide) | 223 | 6200 |
| Poly(ethylene terephthalate) (PET) | 346 | 11300 |
| Poly(Bisphenol F carbonate) | 407 | 13600 |
Glass Transition Temperature vs Cohesive Energy
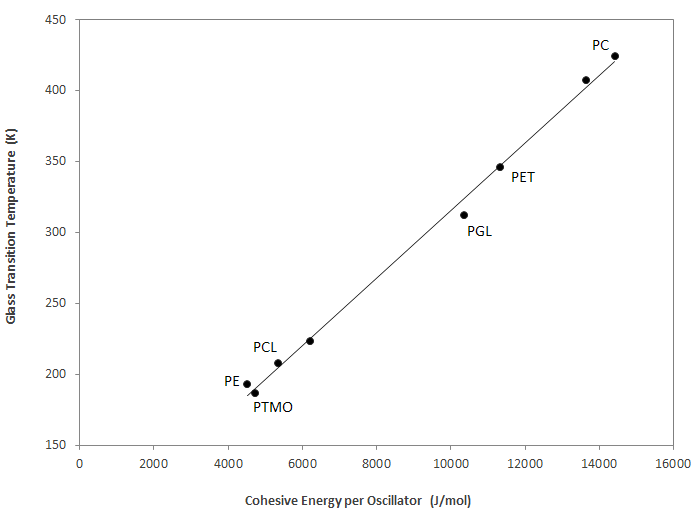
Glass Transition Temperature vs Cohesive Energy
References and Notes
- A.F.M. Barton, CRC Handbook of Polymer-Liquid Interaction Parameters and Solubility Parameters, CRC Press, Boca Raton, 1991.
- U. T. Kreibich and H. Batzer, Die Angewandte Makromolekulare Chemie 83, Nr. 1281, 57-112, (1979) and 105, Nr. 1661, 113-130, (1982)
- Batzer and Kreibich normalized the cohesive energy to the number of structural elements, capable of independent motions, Nosc.