Kelley-Bueche Equation
And Effect of Plasticizers on
Glass
Transition Temperature
It is well known that the glass transtion temperature of an amorphous polymer is reduced by the addition of low-molecular weight miscible compounds such as plasticizers and residual monomer. A low molecular weight compound also causes the glass-rubber transition to occur over a wider range of temperatures than the unplasticized polymer.
In practice, one would like to be able to predict the Tg of a miscible blend like a polymer and plasticizer from the properties of the components. A number of approaches have been proposed to estimate glass transition temperatures of mixtures from the thermo-physical properties of the pure components. All these relationships have in common that they are based on the assumption of additivity and continuity of certain properties at the Tg. One of the most popular equations for predicting glass transition temperatures of amorphous mixtures is the Kelley-Bueche equation (1961):(2)
Tg,mix ≈ ∑i [φi · Tg,i · Δαi] / ∑i [ φi · Δαi]
where Tg,mix and Tg,i are the glass transition temperature of the mixture and of the components, φi is the volume fraction of component i and Δαi(Tg,i) is the change of the coefficient of thermal expansion (CTE) when crossing from the glass to the liquid (rubber) state.
The Kelley-Bueche equation is widely used to describe the effect of plasticizers on the Tg of polymers. However, the relation can only be applied to mixtures where the plasticizer is completely miscible with the host polymer. Furthermore, the equation is only applicable to blends with weak polymer-plasticizer interaction, i.e. van der Waals forces. If specific interactions between the polymer and plasticizer are present, the equation above has to be modified to account for these interactions.(4)
Typically, plasticizers are low molecular weight compounds having a very low Tg. In some cases, a miscible high(er) molecular weight compound of low Tg can be used as a platicizer.
The classic example of a polymer-plasticizer blend is PVC-Phthalate. Unfortunately, these blends show an unusual behavior above 40 % plasticizer content. For this reason, the calculation should be restricted to blends with less than 40 percent plasticizer.
| Weight Fraction DMP | Exper. Tg (K)(5) | Predicted Tga,c (K) | Predicted Tgb,c (K) |
| 0 | 357 | 357 | 357 |
| 0.03 | 343 | 344 | 346 |
| 0.07 | 328 | 329 | 333 |
| 0.15 | 307 | 303 | 309.5 |
| 0.20 | 294 | 290 | 297 |
| 0.275 | 280 | 272 | 279.5 |
| 0.375 | 255 | 254 | 261 |
Glass Transition Temperature of PVC-DMP blends
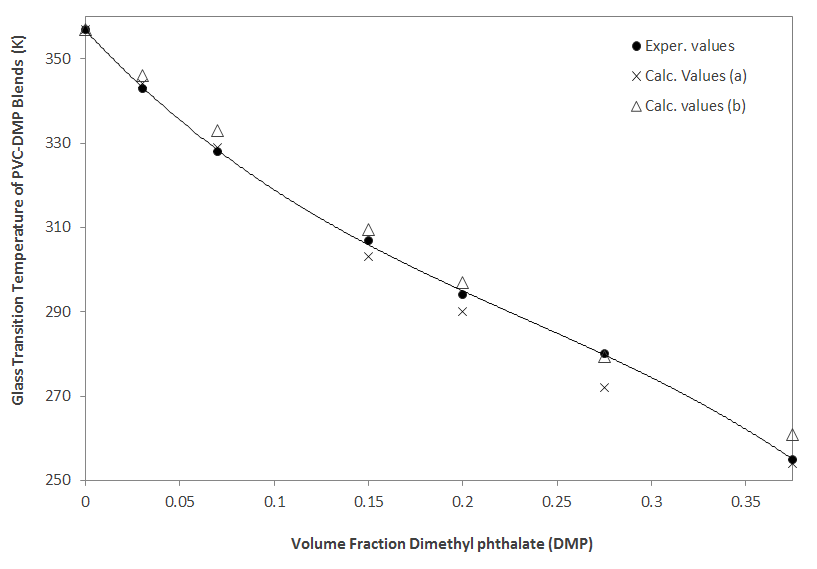
Glass Transition Temperature of PVC-DMP blends
References & Notes
If the polymer is semi-crystalline, the plasticizer also reduces the melting temperature and / or
the degree of crystallinity.- F.N. Kelley and F. Bueche, J. Polym. Sci., 50, 549 (1961)
- J. R. Fried, Polymer Science and Technology, 1st Edition, New Jersey 2003
- X. Lu and R.A. Weiss, Macromolecules 25, 3242 - 3246 (1992)
- Scandole et al., Polymer Bulletin 6, 653-660 (1982)