Flory-Fox Equation
The Flory-Fox equation(1,2) is the most popular empirical equation that relates the number-average molecular weight, Mn, to the glass transition temperature, Tg,
Tg(Mn) ≈ Tg,∞ - K / Mn
Another simple empirical formula for the prediction of the molecular weight dependence of the Tg has been developed by Fox and Loshaek:3
Tg(Mn) ≈ Tg,∞ - K / (Mn Tg,∞2)
where Tg,∞ is the limiting value of the glass transition temperature at very high molecular weight, Mn is the number-average molecular weight and K is a constant for a given polymer that is related to the free volume present in the polymer.6 This dependence can be explained with the free volume theory of the glass transition;(3) due to the greater mobility of the chain ends, the free volume increases with the number of chain ends in a given volume, i.e. it increases with decreasing molecular weight. Loshaek and Fox found that the Tg,∞ is linear proportional to the free specific volume v:
(vg - vg,∞) / (Tg - Tg,∞) = const.
If this slope together with the slope of the v-T curve of the liquid monomer and the liquid polymer of infinite chain length is known, the Tg - Mn
relationship can be predicted, that is, the constant K can be estimated from thermo-physical properties that do not depend on the molecular weight.3,6
Some representative
values of the Flory-Fox parameter for three common polymers are given in the Table below.
| Polymer | Exper. K Values | AVG Predicted K Values |
| Polystyrene | 1.0 10-5 | 1.2 10-5 |
| Polymethylmethacrylate (iso) | - | 1.05 10-5 |
| Polyisobutylene | 0.69 10-5 | 0.65 10-5 |
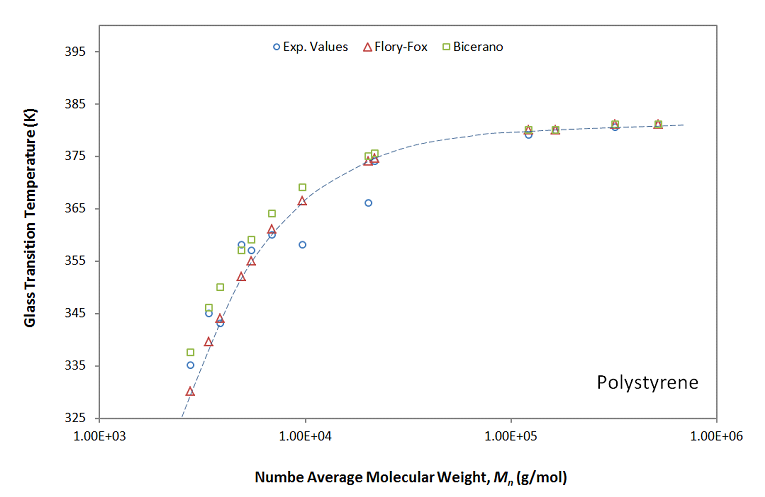
The plot below shows the dependence of the glass transition temperature on the molecular weight for polystyrene, as predicted by the Flory-Fox equation and measured by An, He and Jing (1997). The Tg's have been calculated with the software 3Ps-Tg. The predicted and measured values are in excellent agreement.
Glass Transition Temperature of Polystyrene
References & Notes
- T.G. Fox, and P.J. Flory, Journal of Applied Physics 21, 581–591 (1950)
- T. G. Fox, P. J. Flory, Journal of Polymer Science, 14, 315-319 (1954)
- T.J. Fox, and S.J. Loshaek, Journal of Polymer Science 15, 371 - 390 (1955)
- L. An, D. He, J. Jing et al., Eur. Polym. J., Vol. 33, No 9, 1523 - 1528 (1997)
- Joseph Bicerano, Prediction of Polymer Properties, Marcel Dekker, New York 2002
The factor K is usually obtained by fitting the Flory-Fox equation to experimental data
for a given polymer. If these data are not available, K can be estimated from Bicerano's
equation: K = 0.002715 Tg,∞3.(5)