Light-Controlled Radical Polymerization
Light-controlled radical polymerization (L-CRP) has received increasing attention in recent years.1,2,6,9 This type of living polymerization offers several advantages over conventional controlled radical polymerization including regulated or switchable chain propagation, spatial resolution, short polymerization time and low reaction temperature.1 Light can be not only used for the initiation of free radical growth but also to control the polymerization process when used in combination with UV iniferters.
According to Chen et al.,2,6 all photo-CRPs fall into two categories concerning the photochemical reaction producing the propagating species: (a) intermolecular photochemical processes and (b) photoredox processes. The former proceeds via light-induced homolytic bond cleavage or via intermolecular energy transfer with subsequent bond cleavage and the latter produces radicals via a single electron transfer between a light-activated photoredox catalyst and a donor or acceptor.2
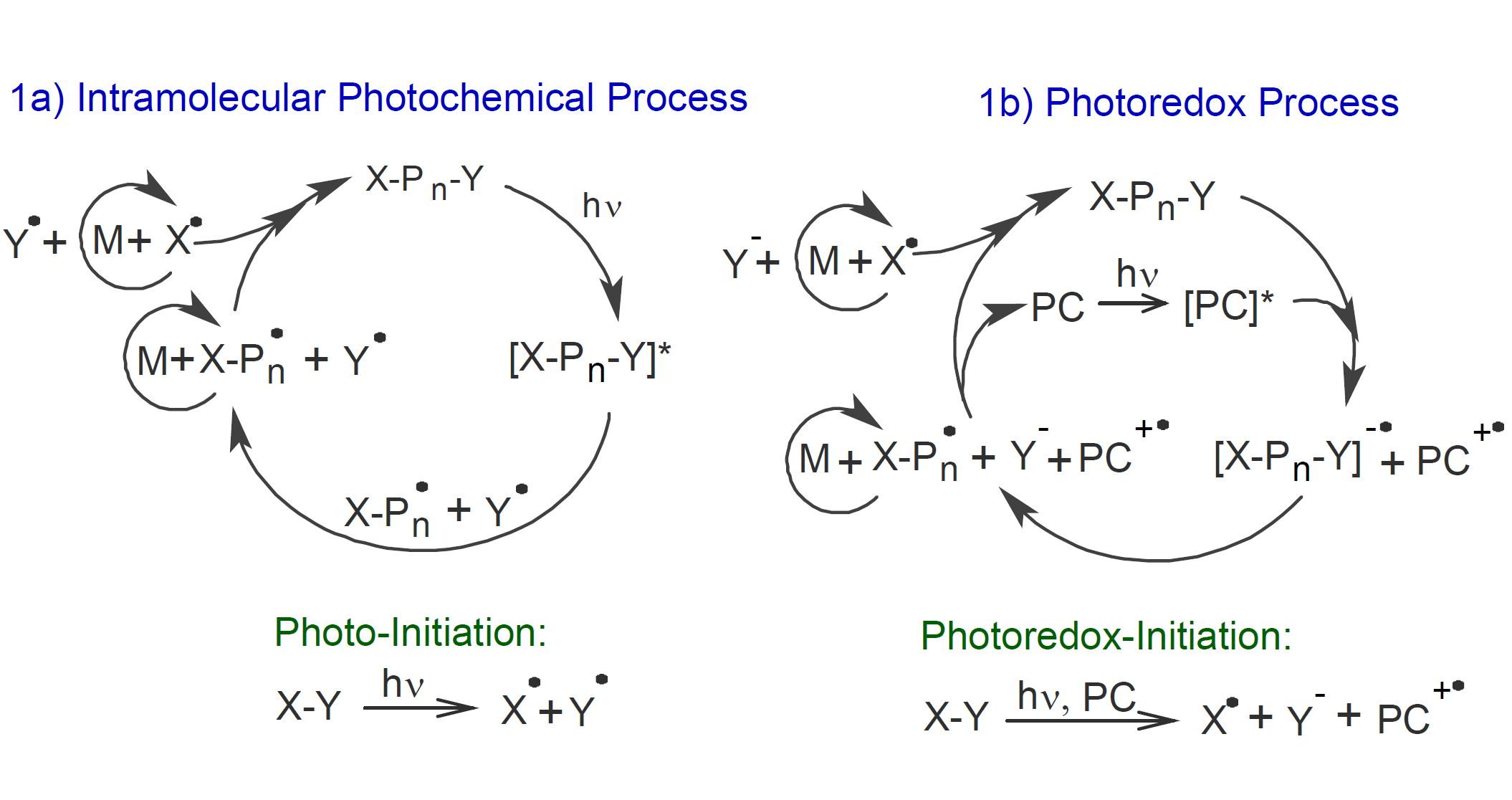
A typical photo-CRP based on an intramolecular photochemical process is depicted in scheme 1a above. The process starts with photo-initiated homolytic bond cleavage of the excited primary photo-initiator. The produced initial radical then directly undergoes a small number of propagation steps followed by reversible recombination which produces a dormant polymer that will act as a secondary polymeric initiator that can be reactivated by a similar photochemical process. Thus, the light-absorbing species becomes part of the propagating polymer. This method allows for good control of the polymerization without a catalyst if all other requirements of a CRP are met.2
In the case of a photoredox CRP (see scheme 1b above), the light-absorbing species functions as a photoredox catalyst (PC) which undergoes a photo induced single electron transfer (SET) to or from an suitable initiator. This process is also known as oxidative quenching since the PC is no longer in an excited state after oxidation.3 The resulting radical anion then undergoes thermal fragmentation to produce the propagating species that immediately or after a few propagation steps regenerates a macroinitiator and a ground state PC via combination with an anion and SET to the oxidized PC. It is also possible that the propagating radical transfers an electron to another ground state initiator.
A major advantage of a photoredox CRP is that the PC is not incorporated into the polymer chain. Furthermore, no additional radical source such as peroxide or azobis(butyronitrile) is required which minimizes the formation of chain termination events and thus allows for better molecular weight control.
To achieve good control of the polymerization process in a photoredox-CRP, the reaction of the initiator fragments with the anion and PC radical should be reversible and highly efficient. This is typically not the case with traditional photo-initiated polymerization where the growth radicals irreversibly terminate when the light source is removed.
Photoinitiation can be applied to almost all types of living polymerizations including ATRP, NMRP and RAFT. It enables the L-CRP to proceed under mild reaction conditions.1 Some of the most common L-CRPs will be briefly discussed below.
Photo-CRP Using Iniferters
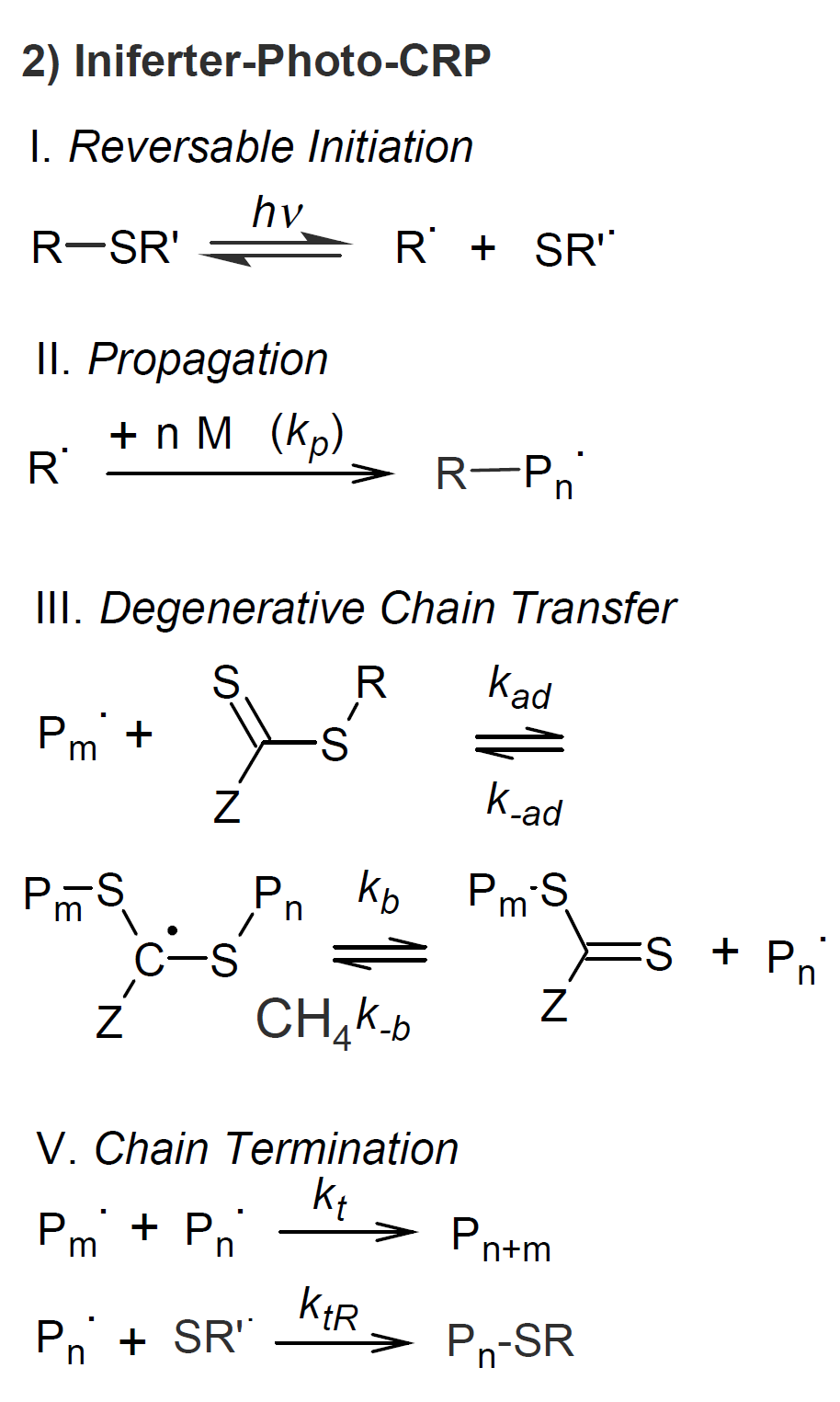
One of the oldest photo-CRP methods is based on thio compounds such as dithiocarbonates and disulfide (see scheme 2). Otsu et al. observed that in the presence of light, dithiocarbonate end groups undergo reversible dissociation from vinyl polymers such as polystyrene and polymethyl methacrylate.4 It was postulated that these iniferters16 undergo bond cleavage under irradiation to produce R· and RS· radicals that not only initiate polymerization but also undergo degenerative chain transfer with other iniferters that were not cleaved by light. Furthermore, the sulfur radicals can recombine with a growing chain end radical to regenerate a dormant macroiniferter. This process is very similar to RAFT. However, unlike RAFT, this type of polymerization did not yield polymers with low polydispersity and only limited control over the chain architecture was achieved.4 A main problem is that dithiocarbamates do not yield inert stable radicals since they dimerize to form dithiuram, whereas CRP iniferters such as TEMPO or TIPNO do not dimerize.5
Nitroxide-mediated Photopolymerization
Another CLP suited for light initiation is nitroxide mediated radical polymerization (NMRP). The mediators used in this type of CLP are alkoxyamines such as TEMPO which undergo homolysis of the C-O bond when irradiated in the presence of a photosensitizer. In many cases the sensitizer is chemically attached to the alkoxyamine. Irradiation of the chromophore followed by intramolecular energy transfer to the alkoxyamine induces homolytic N-O bond cleavage. With the exception of the described initiation step, the mechanism of this type of CLP should be very similar to conventional NMRP. Note this type of photo- CRP is still in development, meaning, so far, living polymerization by this mechanism has been shown for only a few monomers and in many cases, it yielded a broader polymer molecular weight distribution than with conventional NMRP.2, 11,12
Photo-RAFT with Exogenous Photoinitiators
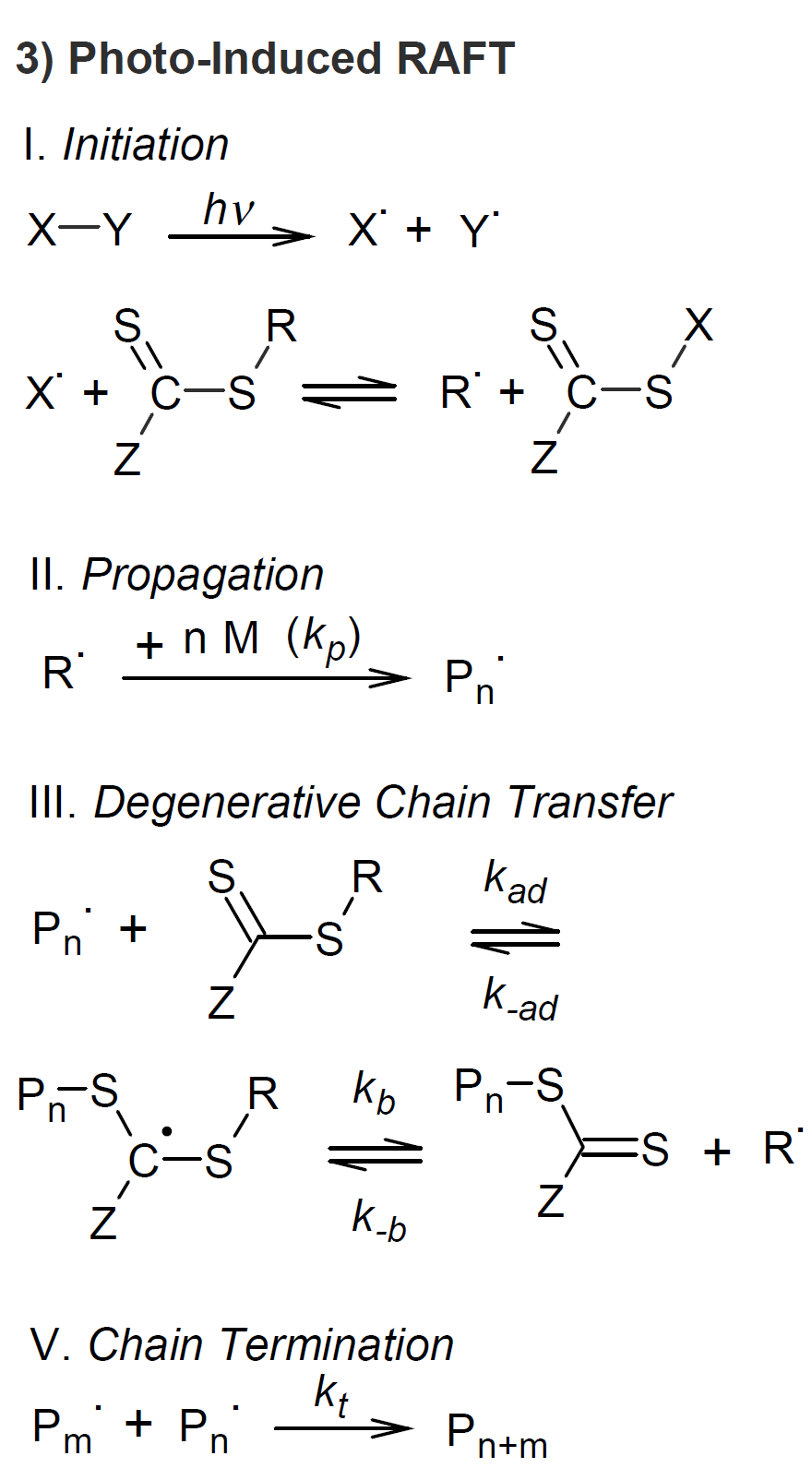
Photo-RAFT is one of the post popular light-controlled CRPs. In this variant of RAFT, the photoinitiator replaces the thermal initiator (see scheme 3). Unlike iniferter-CRP, the activation is not reversible. In other words, in a photo-induced RAFT polymerization, there is no reinitiation step of irreversibly terminated polymer chains. Thus, within each RAFT cycle a fraction of chains stop growing. However, this event is very unlikely, since only a small number of primary radicals are generated during each cycle due to a low irradiation intensity ensuring a low concentration of growing chains, i.e. most polymer chains are in a reversible dormant state. A major advantage of photo-RAFT is that it can be conducted at room temperature without an elaborate degassing procedure due to the high potential for oxygen tolerance of this type of RAFT.6
Direct Photodissociation RAFT
Another type of photo-RAFT is based on the photo-induced homolysis of a RAFT agent. The reaction cycle starts with the photo excitation of the photo-RAFT agent with UV or visible light which then undergoes homolytic cleavage to give directly the initiated radical R·, which replaces the conventional radical produced from an added initiator. Thus, the RAFT agent functions as both a photinitiator and a chain transfer agent, called photiniferter.6
Photoinduced ATRP
Another CLP that was modifed for light initiation is atom transfer radical polymerization
(ATRP). This method requires a special type of photoredox catalyst that can replace the transition metal complex.
The use of these novel catalysts makes the CRP process more robust
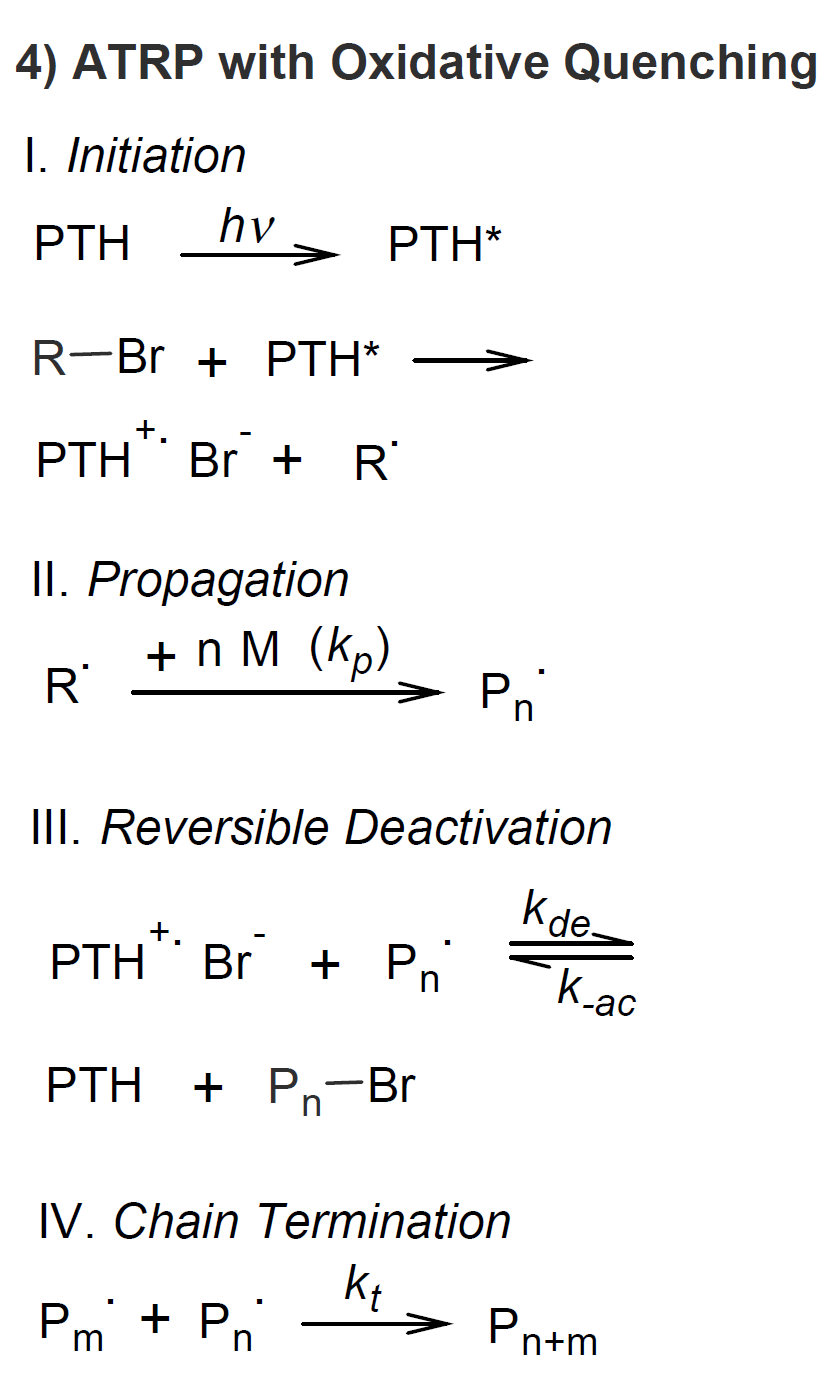
since deoxygenation is not required.9 An important breakthrough was achieved by Fors and Hawkers7 in 2012, who successfully conducted ATRP with tris(2-phenylpyridine)iridium) [Ir(ppy)] photoredox catalyst. Two years later, Treat et al.8,10 reported
a photo-ATRP that uses phenyl-phenothiazine (Ph-PTH), a small molecules photosensitive redox catalyst, that was successfully employed in the controlled photo-polymerization of methacrylates.
Spectroscopic analysis of the polymers synthesized by this method showed no significant difference to polymers produced by traditional ATRP.9
The most common mechanism of photo-ATRP is based on oxidative quenching of
a photo catalyst
(see scheme 4); the reaction cycle starts
with the photo-excitation of the photo catalyst with UV or visible light, followed by a single
electron transfer to an alkyd halide initiator which produces a carbon-centered radical and a photo catalyst radical
cation and bromide anion complex. The former starts the vinyl polymerization whereas the latter acts as a deactivator
that facilitates reversible capping of the propagating polymer via a redox reaction.
Recently, it has been shown that a CRP can be also achieved by a reductive quenching pathway.2.9 In this type of photo-CRP, electron-deficient organic dyes are employed in combination with electron donors.
A major focal point of recent research is the development of more efficient photocatalysts. A large number of different families of organic polycyclic and heterocyclic compounds have been investigated and found suitable for photo-CRP. This research was inspired by the large number of dyes developed in the past and is still ongoing.9,10
References, Notes & Further Reeadings
A. Anastasaki, V. Nikolaou, Q Zhang, et al., J. Am. Chem. Soc. 136, 1141−1149 (2014)
M. Chen, M. Zhong, and J.A. Johnson, Chem. Rev. 116, 17, 10167-10211 (2016)
Note, the majority of reported photoredox CRPs are based on oxidative quenching whereas reductive quenching is much less common.
T. Otsu, M. Yoshida, Makromol. Chem., Rapid Commun., 3, 127−132 (1982)
Krzysztof Matyjaszewski, Macromol. Rapid Common. 26, 135-142 (2005)
X. Tian, J. Ding, B. Zhang, et al., Polymers, 10, 318 (2018)
B.P. Fors and C.J. Hawker, Angew. Chem., Int. Ed., 51, 8850−8853 (2012)
N.J. Treat, H. Sprafke, J.W. Kramer et al. J. Am. Chem. Soc. 136, 16096−16101 (2014)
E.H. Discekici, A. Anastasaki, J.R. de Alaniz, & C.J. Hawker, Macromol., 51, 7421 (2018)
J.T. Trotta, and B.P. Fors, Synlett 27, 702-713 (2016)
Y. Guillaneuf, D. Bertin, D. Gigmes, D.L. Versace, J. Lalevee, J.P. Fouassier, Macromol. 43, 2204−2212 (2010)
E. Yoshida, Colloid Polym. Sci. 287, 767−772 (2009) & 288, 73−78 (2010)
S. Dadashi-Silab, X. Pan, and K. Matyjaszewski, Macromol. 50, 7967-7977 (2017)
E. Adrezejewska, K. Grajek, MOJ Poly. Sci., 1(2), 58-60 (2017)
Q. Ma, J. Song, X. Zhang, Y. Jiang, L. Ji & S. Liao, Nature Com. 12:429 (2021)
Chain transfer agents such as dithiocarbamates are often refered to as iniferters because they act as initiators, chain-transfer agents and terminators.