Group Transfer Polymerization
(Silicon-Mediated Polymerization)
Group transfer polymerization (GTP) was invented at DuPont over 30 years ago.1,2 It is a popular method to control the molecular weight and molecular weight distribution of acrylic polymers during chain growth. Like other living polymerization methods, GTP allows for the preparation of polymers with low polydispersity and with well-defined architecture such as block copolymers and and star-shaped polymers. However, unlike conventional anionic polymerization, methyl methacrylate (MMA) can be polymerized at above ambient temperature. Thus, the temperature can be controlled with simple water-cooled reflux condensers, rather than with more expensive refrigeration units. Furthermore, the process typically uses initiators and catalysts of low toxicity which can be readily removed from the product by filtration,2 and thus can be reused and are not a source of undesirable discoloration or odor. A major drawback of GTP is the sensitivity of the catalysts to protonic impurities (moisture, alcohol etc.) which renders the initiator inactive and causes loss of molecular weight control. Thus, to achieve low dispersity and high molecular weights, all ingredients including monomer, solvent and catalyst have to be carefully purified.4,5
DuPont's GTP process uses tetracoordinate organo-silicon compounds (silyl ketene acetals) like 1-methoxy-1-(trimethylsiloxy)-2-methylprop-1-ene (MTS) as initiator and a nucleophilic anion or Lewis acid as catalyst (referred to also as co-catalyst).2 The Lewis acid most likely functions as an activator by coordination with the carbonyl oxygen of the (meth)acrylate which requires relative large amounts (10% of monomer).12 The co-catalyst also allows for better control over chain growth.
Similar to ordinary living anionic polymerization, the number of growing polymer chains equals the number of initiator molecules added. Chain growth stops when the monomer is completely depleted. But like other living polymerization methods, it can be restarted by addition of more monomer. In the case, a different type of monomer is added, the second growth stage yields block copolymers.
Chain growth is started by intermolecular Michael addition of the ester enolate group of a silyl ketene acetal (SKA) to the vinyl group of a monomer catalyzed by a nucleophilic anion or Lewis acid.9 Early studies indicated that the trimethylsilyl group (TMS) remains with the growing chain center during polymerization which is only possible if the TMS group is transferred to the incoming monomer.3,6,7 A possiible mechanism of TMS transfer is shown below. The TMS transfer can be explained by a concerted rearrangement of double bonds. The process is repeated in each vinyl addition step. (Hence the name GTP)
Associative Group Transfer Mechanism
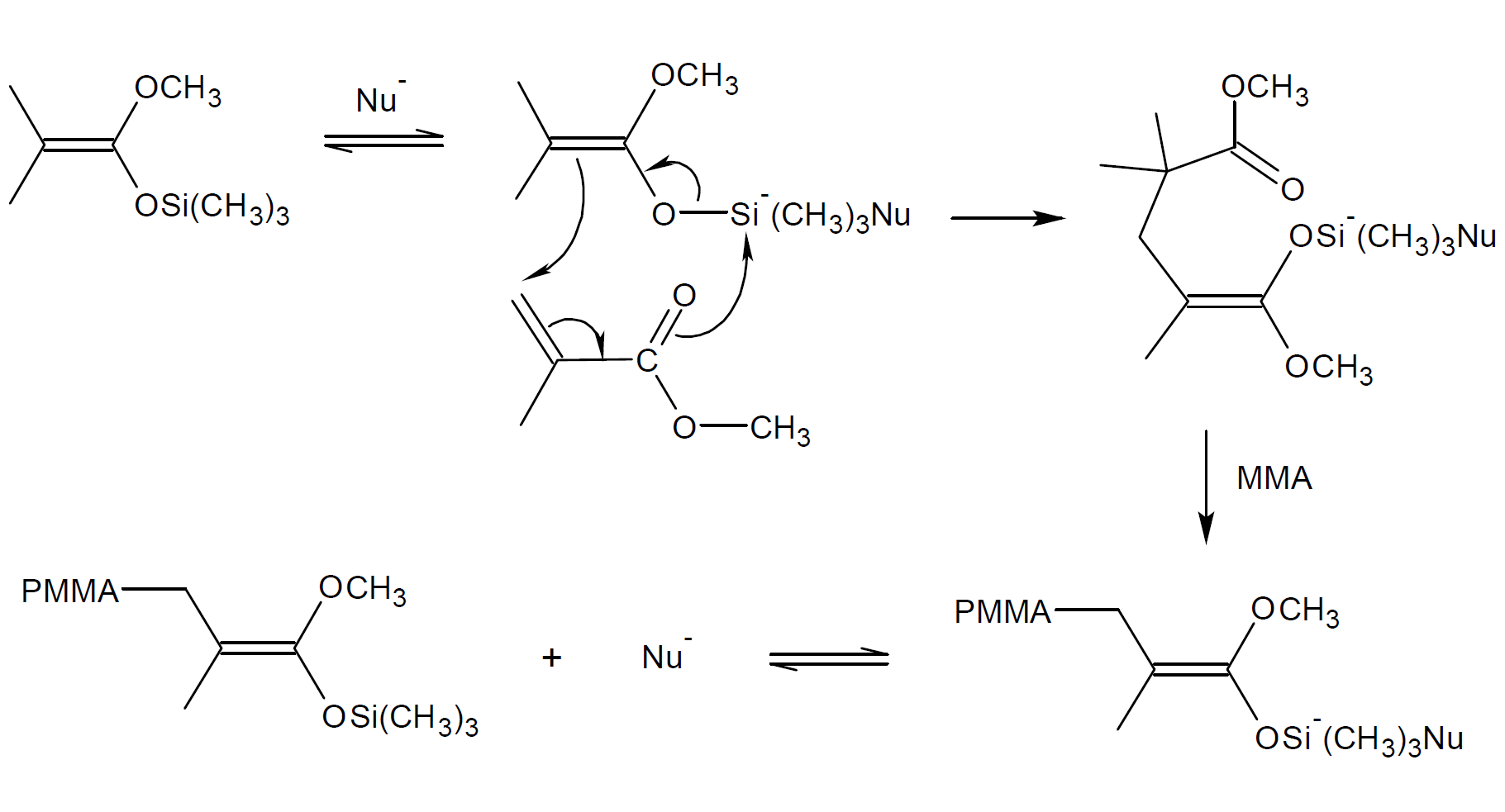
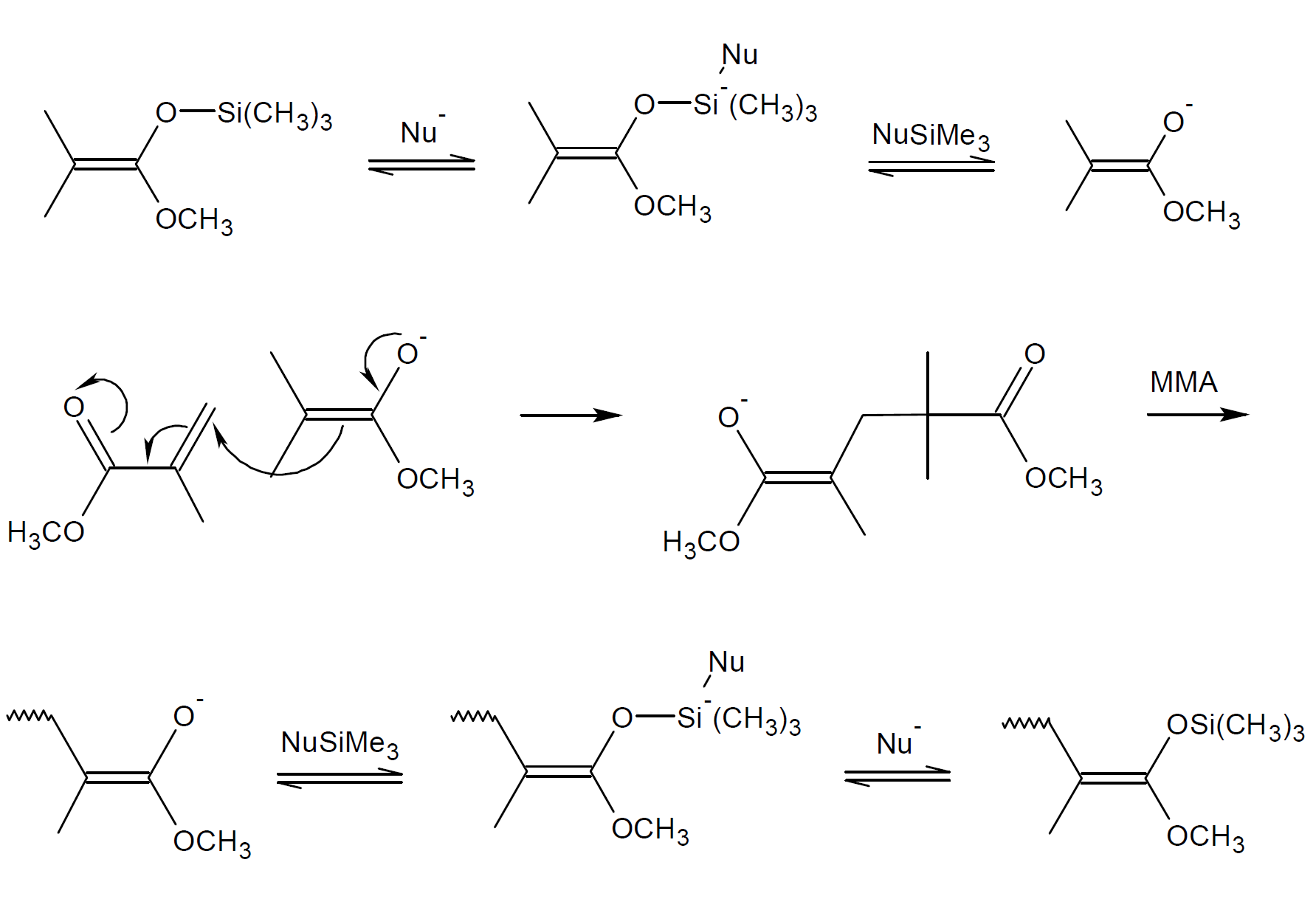
The mechanism above was later disputed because it excludes transfer of trimethylsilyl groups (TMS) to other (dormant) chains. Such a transfer, however, was experimentally observed.3 Webster and coworkers found strong evidence for a dissociative anionic process.8 They suggested that the growth centers are ester enolate anions to which vinyl monomers are added (see mechanism below). In other words, the propagation process is the same as that of conventional anionic polymerization. However, a certain portion of the anionic end groups are reversibly complexed with silyl ketene acetal (SKA). The trapping and releasing of active growth sites by trimethylsilyl groups (TMS) reaches quickly an equilibrium whereby only a small number of chains are active. It allows both the dormant and active chains to grow in a similar fashion. The dissociative mechanism also explains why too much catalyst greatly reduces or stops the GTP process because a large excess of catalyst would shift the equilibrium to the dormant species.
Dissociative Group Transfer Mechanism
Although GTP was developed for living polymerization of methacrylates, other monomers such as alkyl acrylates, N,N-dimethylacrylamide, acrylonitrile, methacrylonitrile, and vinyl ketones can be polymerized as well.5,6
Initially it was believed that both initiator and catalyst have to be soluble to some extent in the monomer or polymerization solvent to start and to keep the GTP polymerization process going. It has been shown, however, that a heterogenous GTP process wherein the initiator and/or the catalyst are chemically attached (grafted) to an insoluble support material is feasable as well. Such a polymerization process facilitates both separation and recovery of the "living" polymer and of the initiator and catalyst.2
References
O.W. Webster, US Patents 4681918 & 4711942A, Living Polymers and Process for Their Preparation, DuPont 1987
F.P. Boettcher, I.B. Dicker, R.C. Ebersole, W.R. Hertler, US Patent 4940760A, Group Transfer Polymerization Process Employing Supported Initiators, DuPont 1989
O.W. Webster, J. Poly. Sci.: Part A: Poly. Chem., Vol. 38, 2855-2860 (2000)
E. Kassi, M.S. Constantinou, C.S. Patrickios, European Polymer Journal 49, 761-767 (2013)
D.Y. Sogah, W.R. Hertler, O.W. Webster, and G.M. Cohen, Macromolecules, 20 (7), 1473-1488 (1987)
O.W. Webster, W.R. Hertler, D.Y. Sogah, W.B. Farnham, and T.V. RajanBabu, J. Am. Chem. Soc., 105, 17, 5706-5708 (1983)
W.B. Farnham, D.Y. Sogah, Polym. Prep. Am. Chem. Soc. Div. Polym. Chem., 27(1), 167 1(986)
O.W. Webster, Adv. Polym. Sci., Vol. 167, pp. 1-34 (2004)
L. Hu, W. Zhao, J. He and Y. Zhang, Molecules, 23, 665 (2018)
P. Vlcek and L. Lochmann, Prog. Polym. Sci., 24, 793-873 (1999)
-
M. Rikkou, Kalourkoti, O.W. Webster, C.S. Patrickios, Group Transfer Polymerization in Ency. Poly. Sci. & Technol. (2013)
-
A.H.E. Mueller and K. Matyjaszewski, Controlled and Living Polymerizations: From Mechanisms to Applications, Wiley-VCH, Weinheim 2009