Nitroxide Mediated Radical Polymerization (NMRP)
Nitroxide mediated free radical polymerization (NMRP, NMP) is the oldest stable free radical polymerization (SFRP) technique. It allows for the preparation of polymers with narrow molecular mass distribution, predictable molecular weight and composition, as well as well-defined polymer architecture including star, comb and gradient copolymers and polymers with a variable functionality.
NMRP was invented by David Solomon, Paul Cacioli, Glen Waverley and Ezio Rizzardo in 1986.1,2 It is one of the most effective polymerization techniques to achieve a quasi living free radical polymerization and is relative easy to perform since it requires only the addition of a hindered alkoxy amine compound to an otherwise conventional free radical polymerization. In fact, it can be as easily performed as a classical free radical polymerization provided a suitable NMRP agent (chain transfer agent, CTA) has been chosen. Furthermore, NMRP polymerization can be thermally initiated in the absence of an external radical source or a metal catalyst and leads to little to no discoloration.3 On the downside, NMRP is less versatile than the two other controlled radical polymerization methods ATRP and RAFT. Polymers that have been successfuly synthesized using NMP include polystyrene, polyacrylates, polymethacrylates, and polyvinylpyridine.4-7
The active organic compound in the process of nitroxide mediated radical polymerization is a sterically hindered alkoxyamine which possesses a thermally labile alkoxyamine bond (C-ON).8 A number of NMRP agents have been developed and synthesized. One of the most versatile NMP agent is 2,2,5-trimethyl-3-(1-phenylethoxy)-4-phenyl-3-azahexane (Aldrich Prod. No. 700703, "Universal initiator") which can undergo homolytic bond cleavage yielding trimethyl-3-(1-phenylethoxy)-4-phenyl-3-azahexane nitroxide (TIPNO). Another important stable nitroxide radical is 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO, Aldrich No. 426369) and its derivatives which are the most popular and widely used NMP initiators.
| Type | Structure |
| 2,2,6,6-Tetramethyl-1-piperidinyloxy (TEMPO) |
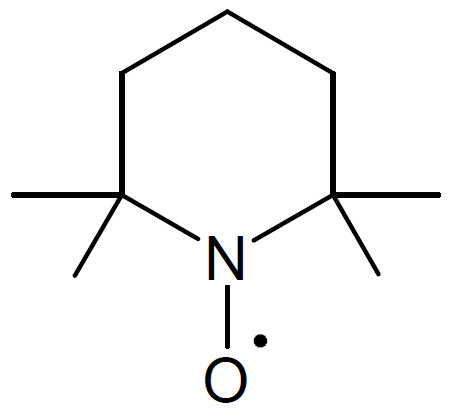 |
| 2,2,5-Trimethyl-4-phenyl-3-azahexane-3-nitroxide (TIPNO) |
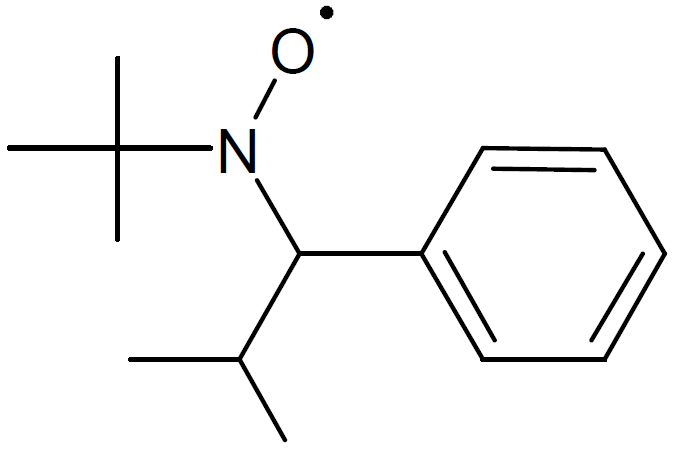 |
| 2,2,5-trimethyl-4-(isopropyl)-3-azahexane-3-nitroxide (BIPNO) |
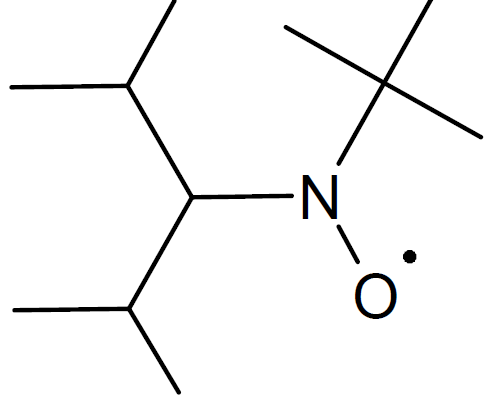 |
NMP Free-radical chain growth can be initiated by a unimolecular fragmentation process or by thermal decomposition of a free radical initiator in the presence of a nitroxide radical.5 Common free radical initiators are azoisobisbutyronitrile (AIBN) and dibenzoyl peroxide (BPO). The initiating radicals react with monomers and then either start chain growth or they react immediately with a nitroxide radical to a nitroxide-trapped alkoxyamine. The chain growth radicals typically undergo only a few propagation steps before they are trapped again by another nitroxide radical.
The trapping and releasing of growing polymer chains, i.e. the addition and fragmentation of nitroxide radical adducts, is a reversible process that rapidly reaches an equilibrium. Shifting this equilibrium to the dormant nitroxide-trapped alkoxyamines (kc >> kd) keeps the concentration of the active chains low, which helps to reduce the probability of irreversible chain termination reactions. A shift of the equilibrium to the dormant species is achieved by an excess of stable nitroxyl radicals.
Mechanism of NMRP Polymerization
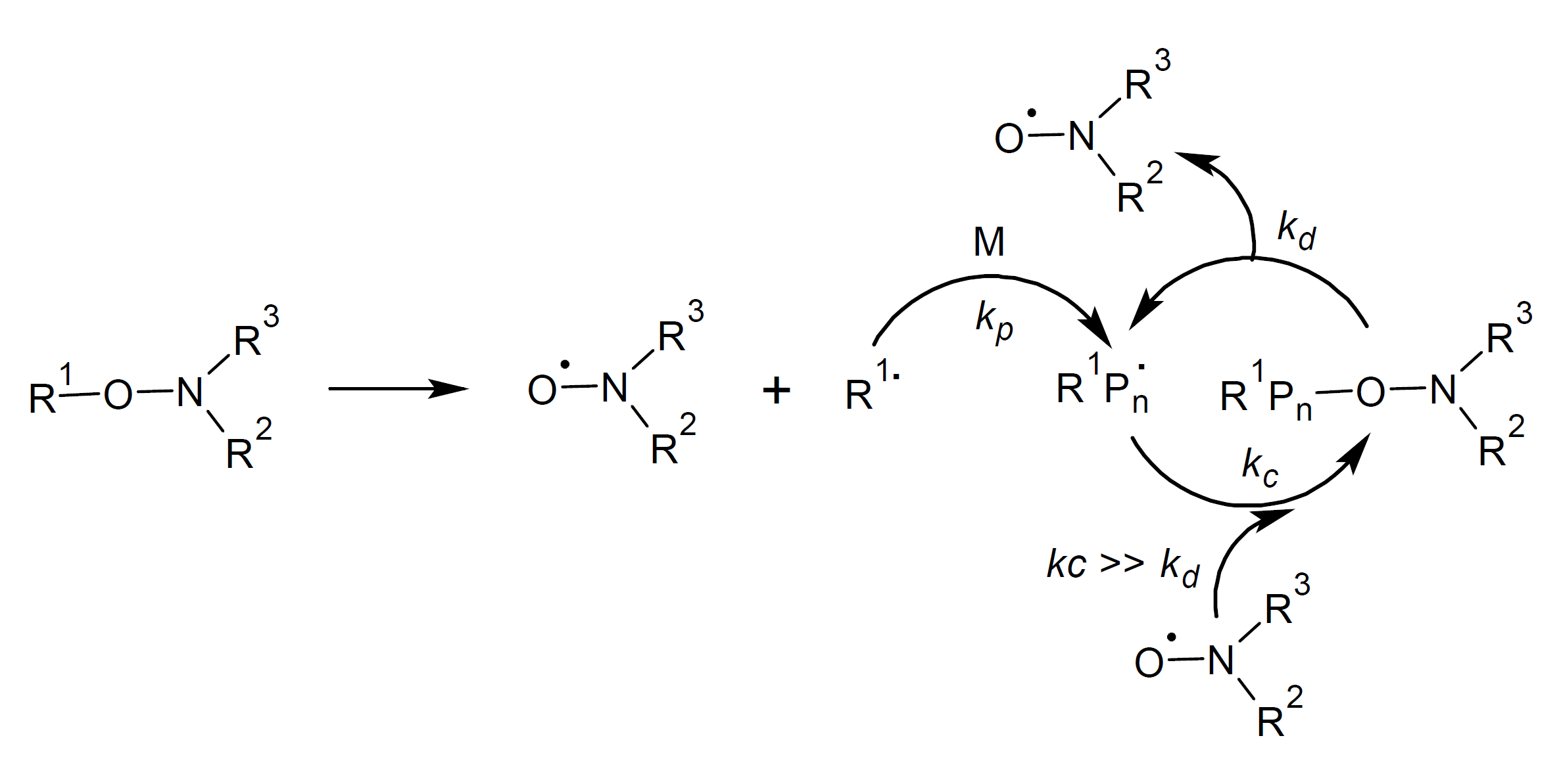
Rapid activation and deactivation allows both the dormant and active chains to grow in a similar fashion. To achieve a high degree of polymerization (DP) and a low dispersity at any time during polymerization, the rate of the reversible radical combination step (reversible termination) should be much higher than that of the propagation step (kc >> kp). The short activation cycle and low concentration of growing polymer chains greatly reduces the number of termination reactions per cycle. However, side reactions such as bimolecular self-termination of growing chain radicals, competing N-OR bond homolysis, chain transfer to solvent molecules, formation of mid-chain radicals and disproportionation termination are not completely suppressed which results in the formation of dead chain ends and increased dispersity. For example, disproportionation reactions observed in the polymerization of methyl methacrylate (MMA) greatly lower the control over the MMA polymerization process which has been an on-going challenge in MMA-NMRP.5
References & Notes
US Patent 4,581,429; Polymerization Process and Polymers Produced Thereby; David H. Solomon, Glen Waverley, Ezio Rizzardo, Wheelers Hill and Paul Cacioli (1986)
Ezio Rizzardo and David H. Solomon, Aust. J. Chem. 65, 945-969 (2012)
N.S. Lee and K.L. Wooley, Block Copolymer Synthesis Using Commercially Available Nitroxide-mediated Radical Polymerization (NMP) Initiator in Controlled Radical Materials Science Polymerization Guide, pp. 31 - 34, Aldrich 2012
A. Fischer, A. Brembilla, and P. Lochon, Macromolecules 32, 6069-6072 (1999)
Johannes Kreutzer and Yusuf Yagci, Polymers 10, 35 (2018)
J.W. Maa, M.F. Cunninghama, K.B. McAuleya, B. Keoshkerianb, M. Georges; Chem. Eng. Sci. 58, 1163-1176 & 1177 - 1190 (2003)
MK. Georges, R.P.N. Veregin, P.M. Kazmaier, and G.K. Hamer, Macromolecules, 26, 2987-2988 (1993)
-
N-Alkoxy based hindered amines are also effective stabilizers in polymer compositions. The hindered nitroxide radicals are produced when hindered amine light stabilizers (HALS) react with hydroperoxides. These compounds are mainly used to inhibit photo-oxidation of polymers.