Controlled Radical Polymerization
Free radical polymerization (FRP) along with ionic and coordination polymerization are the principal synthesis routes for obtaining vinyl polymers. The relatively non-specific nature of free radicals towards vinyl and other unsaturated compounds and its tolerance towards trace impurities makes FRP often the preferred method. On the downside, FRPs typically yield less defined polymers with broader molecular weight distribution than living ionic polymerization techniques. However, the latter methods require carefully controlled systems, i.e. pure reactants and inert solvents to avoid termination reactions whereas the reaction conditions of FRPs are much less demanding. For this reason, a free radical polymerization with living character is very desirable.
Despite its many advantages and wide spread use, the fast chain growth and the occurrence of rapid and irreversible termination reactions makes classical FRP less suited for the synthesis of polymers with well-defined architecture, predictable functionality and low polydispersity. In order to grow such chains, the rate of termination must be much lower than the rate of propagation. This requires short activation-deactivation cycles and very low concentrations of growing polymer chains, because this greatly reduces the number of termination reactions per cycle. In other words, the majority of free-radicals have to be reversibly “trapped” by a suitable trapping agent. However, termination reactions cannot be fully suppressed in a controlled FRP, that is, they only can become very unlikely events if most of the radicals are in a reversible dormant state. To achieve these conditions, the polymer radical concentration must be in the range of parts per billion.1 Furthermore, the rate of initiation has to be much faster than the rate of propagation since all chains have to start to grow essentially at the same time to achieve very low polydispersity.
Controlled FRPs based on reversible activation-deactivation techniques are the most successful quasi-living radical polymerization methods currently known which may be classified into three mechanisms:14 a) dissociation-combination (DC), b) degenerative chain transfer (DCT), and c) atom transfer (AT) (see scheme below).
MechanismS of Controlled Radical Polymerization
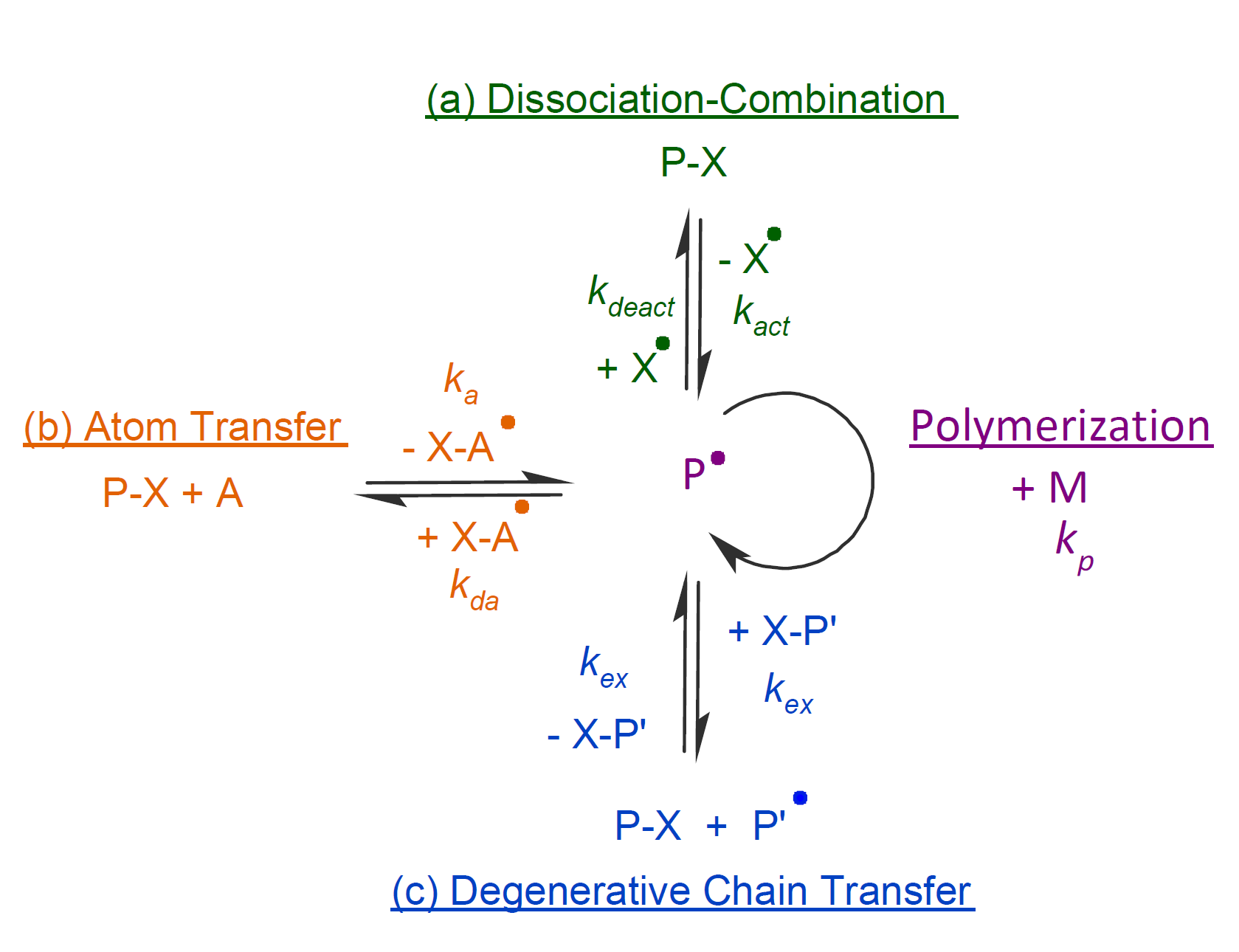
In the dissociation-combination mechanism, capped polymers (P-X) are thermally or photochemically cleaved to polymer radicals (P•) and small persistent molecule radicals (X•) (scheme a), where the formed chain radicals undergo only a few propagation steps before they are trapped again by another trapping radical X•. Suitable capping agents for this mechanism include nitroxide radicals such as 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) and trimethyl-3-(1-phenylethoxy)-4-phenyl-3-azahexane nitroxide (TIPNO) among many other persistent radicals. This type of CRP is known as nitroxide-mediated polymerization (NMP)2,3.
In the degenerative chain transfer mechanism, a polymer radical adds to a double bond of a chain transfer agent, forming a chain extended polymer intermediate (P-X-P') that undergoes fragmentation into a polymer radical (P•) and a capped polymer (P'-X) in a subsequent step. This mechanism is known as reversible addition-fragmentation chain transfer polymerization (RAFT).4 Chain transfer agents that belong to this type include dithioesters, trithiocarbonates, dithiocarbamates, and xanthates.
A third mechanism that allows for quasi-living FRP has been coined atom transfer (AT). In this mechanism, the dormant polymer (P-X) is activated by a halide complex of a transition metal like Cu and Ru which abstracts a halogen atom (typically Cl or Br) from a initiator molecule (initiation) or from a dormant halogen-capped polymer chains (propagation) and thereby generates a free radical whereby the metal complex changes its oxidation state from z to z+1. This type of CRP is termed atom transfer radical polymerization (ATRP)5,6.
All three methods are based on forming a rapid equilibrium between a low concentration of propagating free radicals and a large concentration of dormant polymers which ensures that termination reactions become very unlikely events.
Over the years, numerous other controlled radical polymerization methods have been developed utilizing a variety of capping agents such as nitrogen and carbon compounds,7,8 iodine,9 and organometals such as tellurium, stibine, and bismuth compounds.10-13, 15 Most of these methods are based on a degenerative chain transfer mechanism.
References & Notes
V. Mishra and R. Kumar, Journal of Scientific Research, Vol. 56, 141-176 (2012)
E. Rizzardo, D. H. Solomon, Polym. Bull., 1, 529 (1979)
P. G. Griffiths, G. Moad, E. Rizzardo, D. H. Solomon, Aust. J. Chem., 36, 397 (1983)
J. Chiefari, Y. Chong, F. Ercole, J. Krstina, J.Jeffery, T. Le, R. Mayadunne, G. Meijs,
C. Moad, G. Moad, E. Rizzardo, S. Thang, Macromolecules 31, 5559 (1998)J.S. Wang and K. Matyjaszewski, J. Am. Chem. Soc. 117, 5614-5615 (1995)
J.-S. Wang, K. Matyjaszewski, Macromolecules, 28, 7901-7910 (1995)
D. Colombani, M. Steenbock, M. Klapper, K. Mullen, Macromol. Rapid. Commun. 18, 243 (1997)
B. Yamada, Y. Nobukane, Y. Miura. Polym. Bull. 41, 539 (1998)
M. Miyamoto, M. Sawamoto, T. Higashimura, Macromolecules 17, 265−268 (1984)
K. Takagi, A. Soyano, T.S. Kwon, H. Kunisada, Y. Yuki, Polym. Bull. 43, 143 (1999)
S. Yamago, J. Polym. Sci., Part A: Polym. Chem. 44, 1-12 (2006)
S. Yamago, B. Ray et al., J. Am. Chem. Soc. 126, 43, 13908 (2004)
S. Yamago, E. Kayahara et al., Angew. Chem. 46, 8, 1304-1306 (2007)
A. Goto, Y. Tsujii, and T. Fukuda, Polymer 49, 5177 - 5185 (2008)
The corresponding CRPs are known as organotellurium-, organostibine-, and organobismuthine-mediated living radical polymerizations.
July 11, 2020 & revised January 1, 2023