Reversible Addition-Fragmentation Chain Transfer Polymerization
Reversible addition - fragmentation chain transfer polymerization (RAFT) is a novel and popular method to control the molecular weight and molecular weight distribution of a free radical polymerization. It allows for the preparation of polymers with well-defined polymer architecture including star, block, brush, comb, and gradient copolymers as well as polymers with a predictable functionality.
RAFT was invented by Tam. P. Le, Graeme Moad, Ezio Rizzardo, and San H. Thang in 1998.1 It is one of the most versatile and effective polymerization techniques to achieve a quasi living free radical polymerization and is relative easy to perform since it requires only an addition of a thiocarbonylthio compound to an otherwise conventional free radical polymerization. In fact, it can be as easily performed as a classical free radical polymerization provided a suitable RAFT agent (chain transfer agent, CTA) has been chosen.2,3,10 Furthermore, a large number of vinyl monomers can be polymerized with good molecular weight control and narrow molecular weight distribution. Not surprising, RAFT has gained great popularity by the scientific community and has been employed to synthesize a broad range of materials including numerous polymers such as poly(meth)acrylates, poly(meth)acrylamides, polyacrylonitrile, polystyrene, polyvinyl acetate, N-vinylpyrrolidone, and several polydienes.3,8
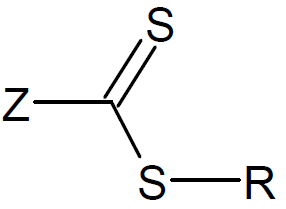
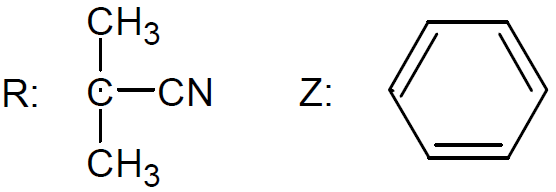
The active organic compound in the process of reversible addition - fragmentation polymerization is a thiocarbonylthio group. The organic compound containing this functional group is commonly called the RAFT agent. A large number of RAFT agents have been developed and synthesized. The majority of these compounds belong to one of the following five classes: dithioesters, xanthates, trithiocarbonates, dithiocarbamates, and dithiophosphonates. Among these, the dithioesters are the most popular and widely used RAFT agents.12
| Type | Structure | Examples (R, Z) |
| Dithioesters |
 |
 |
| Trithiocarbonates |
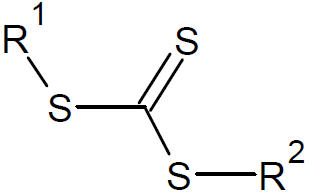 |
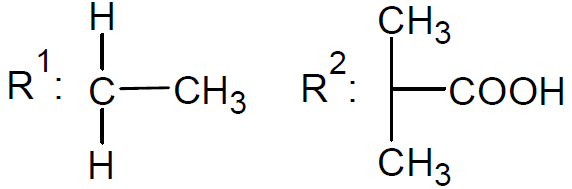 |
| Dithiocarbamates |
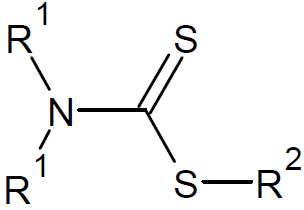 |
 |
| Xanthates |
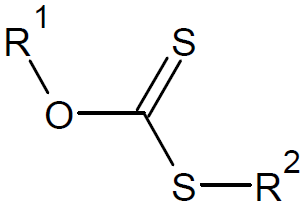 |
 |
Free-radical chain growth can be initiated by any method but is usually started by continuous thermal decomposition of free radical initiators such as azoisobisbutyronitrile (AIBN) or dibenzoyl peroxide (BPO). The initiating radicals react with a monomer unit thereby starting chain growth (I). After a small number of monomers have been added to the initiator radicals (Pn•), the chain radicals are trapped by the thiocarbonyl thio group of the RAFT agent to produce an intermediate radical (II). Fragmentation of the intermediate can either produce the original initiating radical species (Pn•) and the RAFT agent or it may be converted to the polymeric RAFT agent and an organic radical R•. Ideally the reverse reaction during the first addition-fragmentation should be energetically very unfavorable so that only the forward reaction takes place. This is only the case, if R is a much better leaving group than the polymer chain Pn. In practice, a RAFT agent/monomer pair has to be carefully chosen so that the forward reaction (i.e. R• fragmentation) dominates. The free radical leaving group R• must also be able to efficiently reinitiate chain growth (III), otherwise polymerization stops.
The trapping and releasing of growing polymer chains, i.e. the addition and fragmentation of radical adducts, is a reversible process that rapidly reaches an equilibrium (IV). It allows both the dormant and active chains to grow in a similar fashion. To achieve a high degree of polymerization (DP) and a low dispersity at any time during polymerization, the rate of the addition step should be much higher than that of the propagation step, or in other words, there should be less than one monomer added per activation cycle. The short activation cycle and low concentration of growing polymer chains will also greatly reduce the number of termination reactions per cycle (V). However, termination is not fully suppressed, that is, after a short initiation phase, where many radicals undergo bimolecular combination or disproportionation, chain termination becomes a very unlikely event, because most of the remaining radicals are trapped by the thiocarbonyl thio groups of the RAFT agent and thus become dormant. As a consequence, the number of dead chains is solely controlled by the number of radicals initially introduced in the system.
Mechanism of RAFT Polymerization
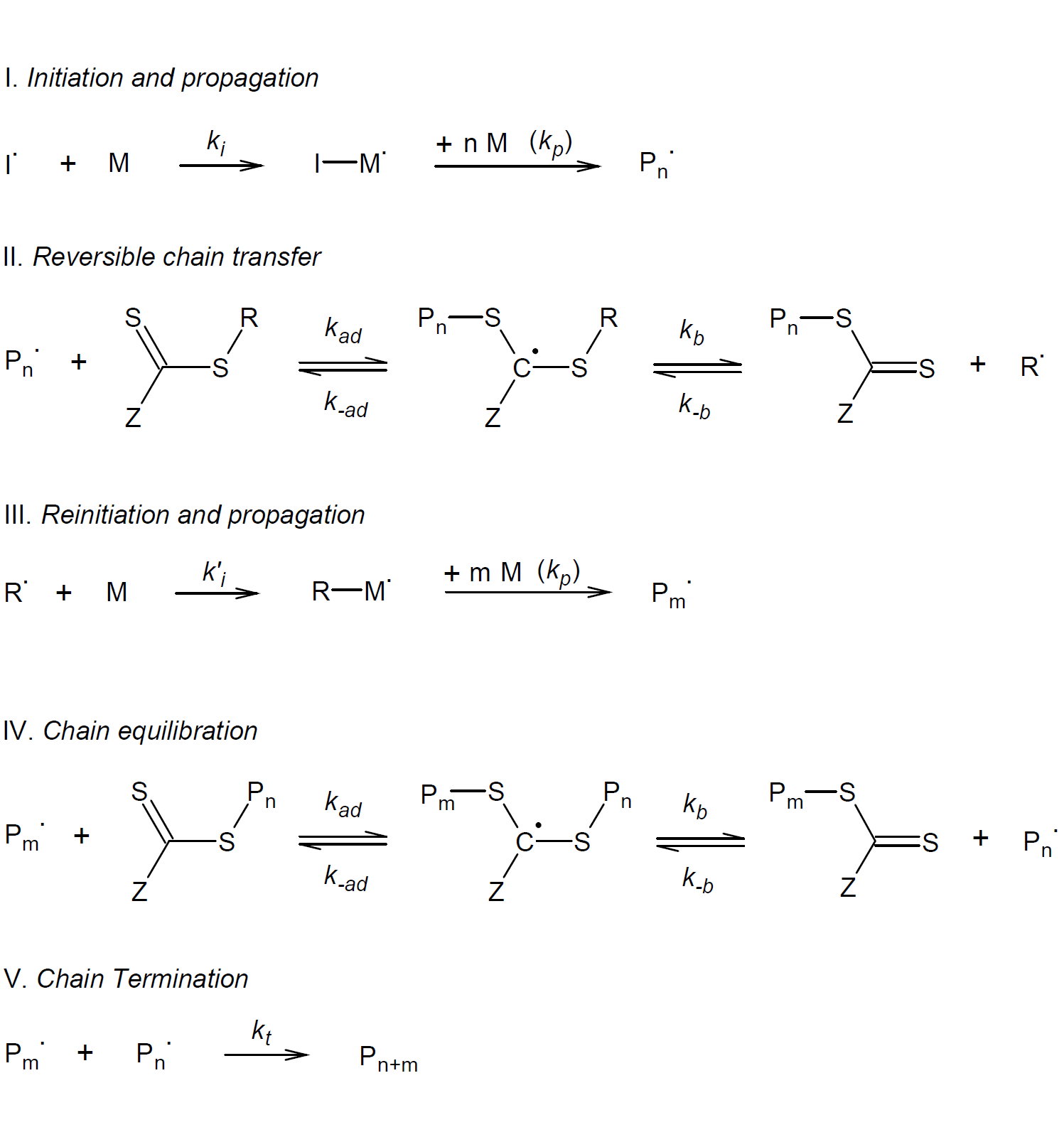
To achieve a living polymerization with a controlled and narrow molecular weight distribution, the C=S bond has to be more reactive to radical addition than the C=C bond of the monomer.8
This is achieved by choosing suitable Z- and R-groups which depend on the type of monomer.
The RAFT monomers can be classified as ′more-activated monomers′ (MAMs) and 'less activated monomers′ (LAMs).
MAMs have a vinyl bond conjugated to another double bond, including those of aromatic rings,
as well as carbonyl and nitrile groups. Monomers that belong to this
class include butadiene, isoprene, styrene,
vinyl pyridine, (meth)acrylates, (meth)acrylamides, maleic anhydride, maleimide, and acrylonitrile. LAMs have a double bond adjacent to an electron-withdrawing group
such as a nitrogen, oxygen, halogen, or sulfur atom with a lone electron pair, or they have saturated carbons attached to the vinyl carbon atoms. Thus, vinyl acetate, N-vinylpyrrolidone, vinyl
chloride, and alkenes are LAMs.
Good control over polymerization of MAMs is achieved with trithiocarbonates (Z = S-alkyl)
or aromatic dithioesters (Z = aryl).6 The most versatile and active RAFT agents are
aromatic dithioesters (Z = aryl). However, the aromatic group may significantly retard the overall rate of polymerization when used in high concentrations.9
It can also be prone to hydrolysis.
However, the very active RAFT agents Z = R (dithioesters) or SR (trithiocarbonates) are not suitable for polymerization of a LAMs because they inhibit the polymerization. A much better choice are less
active RAFT agents such as dithiocarbamates with Z = NR′2, and xanthates with electron withdrawing groups (Z = OR).6-8 The choice of the R group is also important. It must be a good
homolytic leaving group with respect to the propagating radical.
References & Notes
T.P. Le, G. Moad, E. Rizzardo, S.H. Thang , Int. Pat. WO9801478 (1998)
J. Chiefari, Y. Chong, F. Ercole, J. Krstina, J.Jeffery, T. Le, R. Mayadunne, G. Meijs,
C. Moad, G. Moad, E. Rizzardo, S. Thang, Macromolecules 31, 5559 (1998)G. Moad, E. Rizzardo, and S.H. Thang, Aust. J. Chem. 58, 379-410 (2005)
G. Moad, E. Rizzardo, and S.H. Thang, Aust. J. Chem. 59, 669-692 (2006)
G. Moad, E. Rizzardo, and S.H. Thang, Polymer 49, 1079 (2008)
G. Moad, E. Rizzardo, and S.H. Thang, Aust. J. Chem. 62, 1402-1472 (2009)
G. Moad, E. Rizzardo, and S.H. Thang, Material Matters, Vol. 5, No.1 (2010)
S. Perrier, Macromolecules, 50, 19, 7433 - 7447 (2017)
-
Retardation may be attributed to the high stability of the leaving group, i.e. to the tertiary radical stabilized with a phenyl group. The mechanism of retardation is not fully understood.
-
RAFT is a type of reversible deactivation radical polymerization (RDRP) which involves degenerative chain transfer (also called degenerate chain transfer). Two other examples of RDRP are iodine-transfer radical polymerization (ITRP) and telluride-mediated radical polymerization (TERP). According to IUPAC10, the new chain carrier and chain-transfer agent produced by the addition-fragmentation mechanism: P· + XR → PX + R· (IV) must have the same reactivity as the original chain carrier and chain-transfer agent.
RDRPs are only quasi- or pseudo-living polymerizations because they involve some termination reactions, however, with relatively low occurrence. In contrast, a true living polymerization is characterized by the absence of any irreversible termination reaction. -
A.D. Jenkins, R.G. Jones, and G. Moad, Pure Appl. Chem., Vol. 82, No. 2, pp. 483-491 (2010)
-
RAFT agents such as dithiocarbamates are often refered to as iniferters because they act as initiators, chain-transfer agents and terminators.