Hindered Amine Light
Stabilizers (HALS)
One of the most important classes of antioxidant for long-term heat protection of polymers are the so called hindered amine light stabilizers (HALS) which are very effective inhibitors against free radical induced degradation of polymers at low and medium temperatures. This class of amine stabilizers is based on 2,2,6,6-tetramethyl-piperidine derivatives. They are often employed as light stabilizers, particularly for olefins which explains their naming.
HALS can be categorized according to their molecular weight (MW): HALS with low molecular weight of about 200 to 500 g/mole are commonly referred as low MW HALS, while those with a molecular weight of 2000 or higher are referred as high MW HALS. These two classes have different rates of diffusion in the polymer matrix, which is an important factor in protecting polymers from UV radiation. In general, high MW HALS are more effective long-term heat stabilizer than low MW HALS, whereas very low MW HALS do not provide much thermal stability at all. The efficiency of HALS can also be greatly affected by the structure, i.e. side groups, and sometimes by the formulation and surface conditions. To maximize the light stability of plastics, the most suitable HALS structure must be chosen.
Photo-oxidation usually starts at the surface with visible cracks, which leads to rapid degradation of the mechanical properties. In theory, olefins such as polyethylene (PE) and polypropylene (PP) are stable when exposed to sunlight, because both C-C and C-H bonds do not absorb UV radiation from the sun. However, there are always contaminations present like residual catalyst, pigments, and thermal degradation by-products from melt processing of the resins that can initiate photo degradation of any polyolefin when exposed to UV light, which, in turn, causes chain scission and/or crosslinking and eventually surface cracking and loss of mechanical properties.
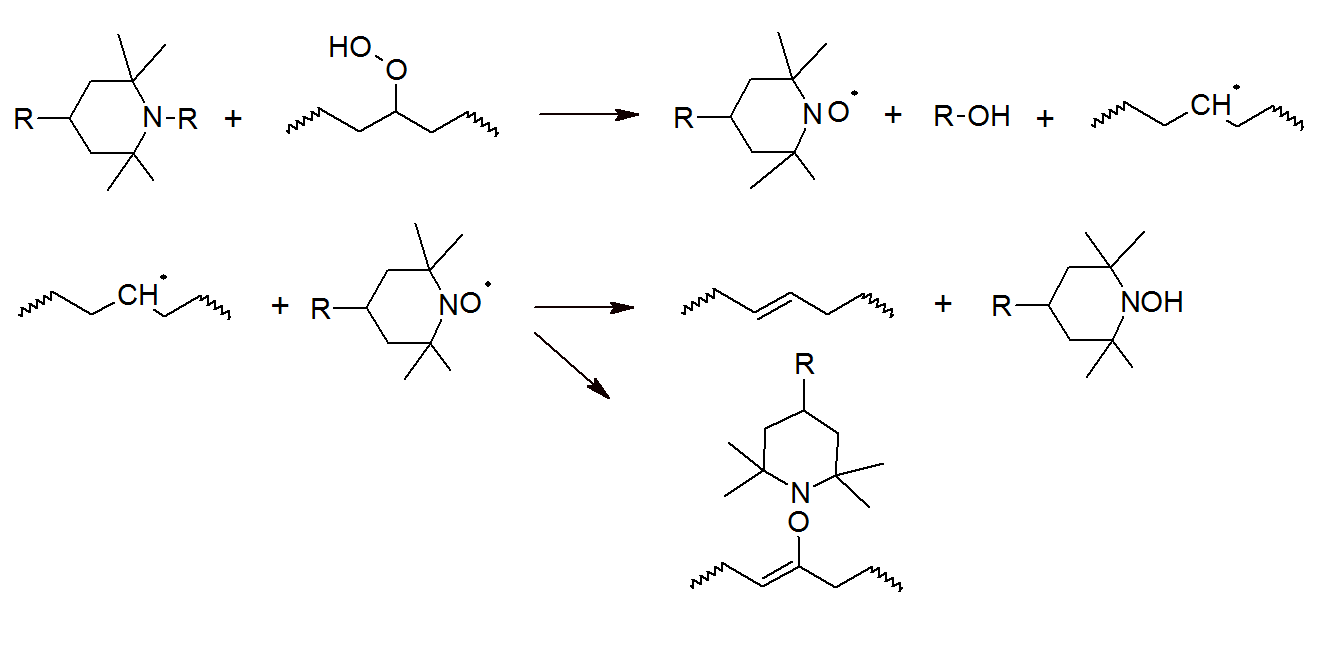
The high efficiency of HALS is based on a complex set of free-radical scavenging reactions, which starts with the oxidation of HALS to nitroxide radicals (RNO·) by reaction with peroxides or hydroperoxides (see figure above), which subsequently react with polymeric alkyl radicals, yielding hydroxylamine ethers which can react with a variety of compounds. New RNO· radicals may be regenerated from the reaction of hydroxylamine ethers with alkylperoxy and acylperoxy radicals (Denisov cycle).
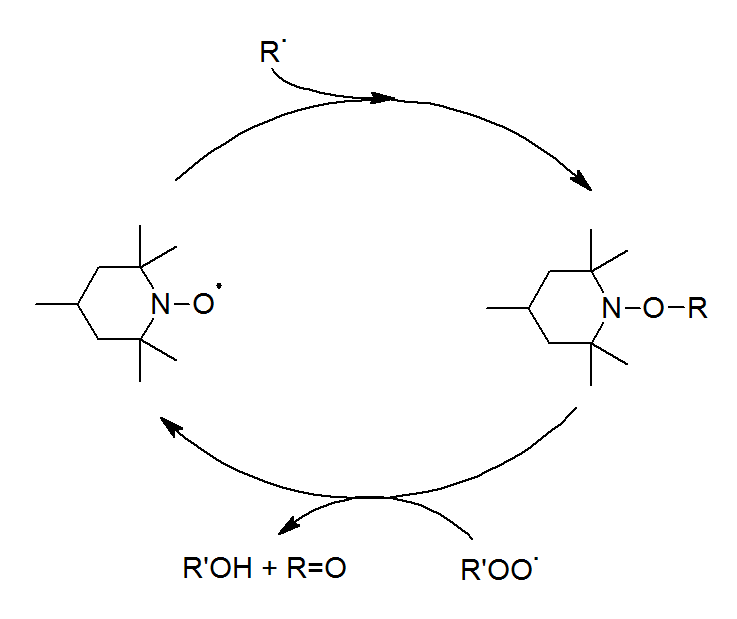
The oxidation of HALS to the NO· radicals is a relatively slow and temperature-dependent reaction. Furthermore, they are not very efficient at elevated temperatures (above 80°C or so), thus, they are not very effective processing stabilizers. For this reasons, HALS are often used in combination with primary and secondary antioxidants. This combination may also show synergistic or antagonistic effects. Due to these unpredictable effects, complex mixtures are often used. For this reason, the study of interactions between stabilizers from different chemical classes is of great importance.
References
- E. N. Step and N. J. Turro, Macromolecules 27, 2529-25 (1994)
- R. Gensler, C.J.G. Plummer, Polymer Degradation and Stability, 67, 195 - 208 (2000)