Ring-opening Copolymerization and Crosslinking of Cyclic Ethers
Cyclic ethers are of significant industrial importance and find applications in many areas including high performance adhesives, inks, coatings and composites. The two most important types of cyclic ethers are oxiranes (also known as epoxides) and oxitanes. These compounds are highly reactive due to the high ring strain and can be copolymerized or crosslinked with a wide variety of nucleophilic and electrophilic compounds having active hydrogen atoms. This includes aliphatic and aromatic amines (both primary and secondary), phenols, carboxylic acids, thiols, and anhydrides. The general reactions of epoxides with these compounds are shown in the scheme below.
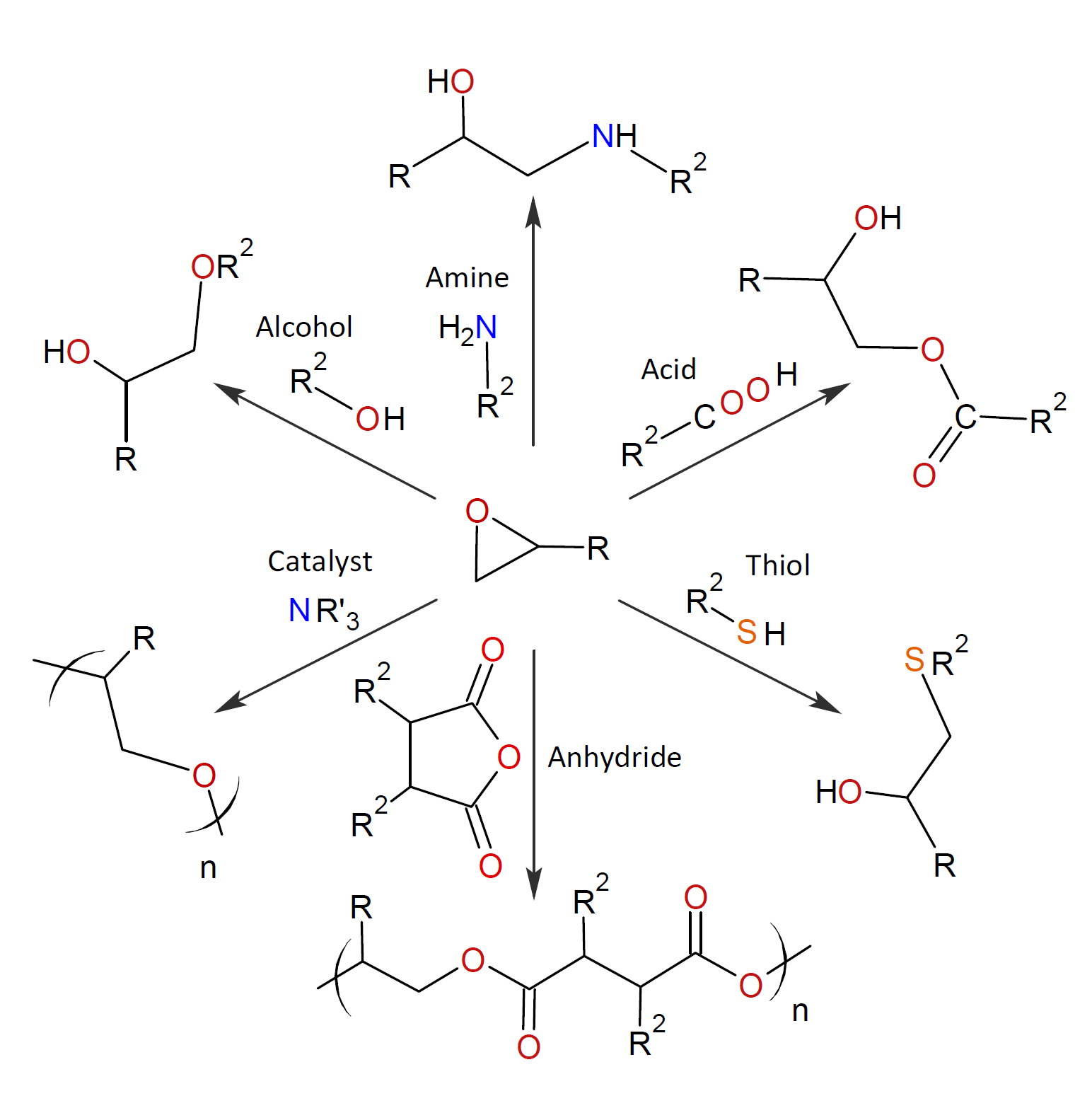
Multifunctional cyclic ethers such as diglycidyl ethers of bisphenol A or F (known as “epoxy resin”) are often cross-linked to achieve optimal performance properties in the final product. This requires co-reactants with three or more active hydrogens per molecule. When copolymerized with multifunctional cyclic ethers, they form a three-dimensional insoluble and infusible network. The choice of co-reactant depends on the required processing characteristics such as viscosity, pot-life, and gel-time and on the performance requirements (chemical, mechanical, thermal, optical, and electrical) and cost.
The three most common co-reactants, also called curatives or hardeners, are multifunctional mercaptans, amines, and anhydrides. The possible (crosslinking) reactions with epoxides are discussed briefly below:
1) Amine-Epoxy Addition Reactions
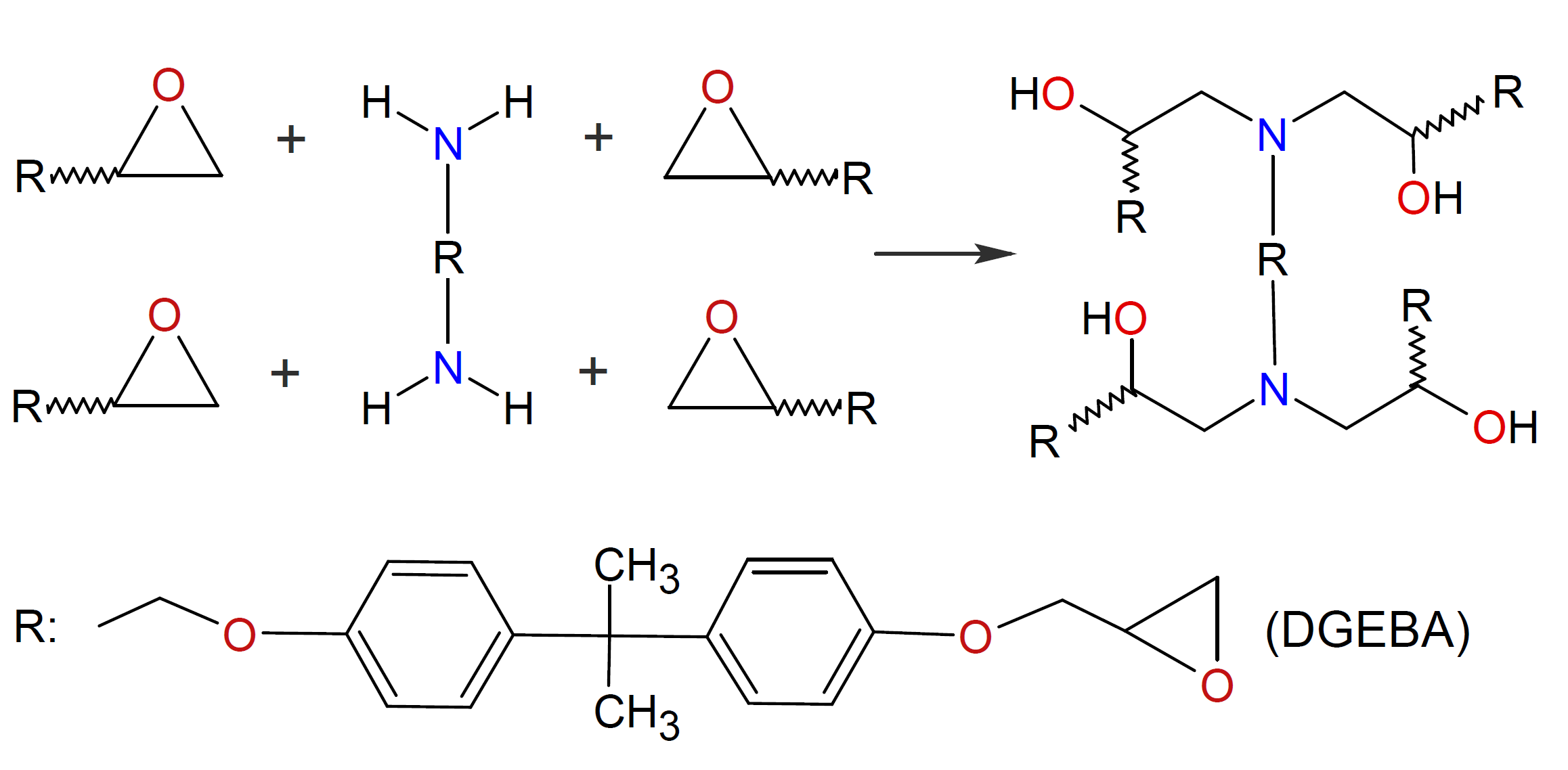
Polyamines are the most common crosslinking agents for diepoxides such as diglycidyl ether of bisphenol A (DGEBA). Depending on the substituents, the amines can be divided into three main groups: aliphatic, cycloaliphatic and aromatic amines. Among these, cycloaliphatic amines are the most reactive ones followed by aliphatic amines whereas aromatic amines are much less reactive due to their weaker nucleophilicity and typically require long cure cycles at elevated temperatures (> 100°C) but yield the highest chemical and thermal resistance properties.
Crosslinking of epoxy resins with amines typically proceeds via nucleophilic addition. In the case of primary amines, the addition reaction yields a secondary amine and a hydroxyl group. The resulting secondary amine then reacts with another oxirane ring, creating an additional hydroxyl group and a tertiary amine. An idealized representation of the reaction between epoxy resin and diamine is depicted in the figure below. In theory, all active amine hydrogens can undergo addition reactions, but in reality, vitrification occurs long before all reactive groups are consumed.1
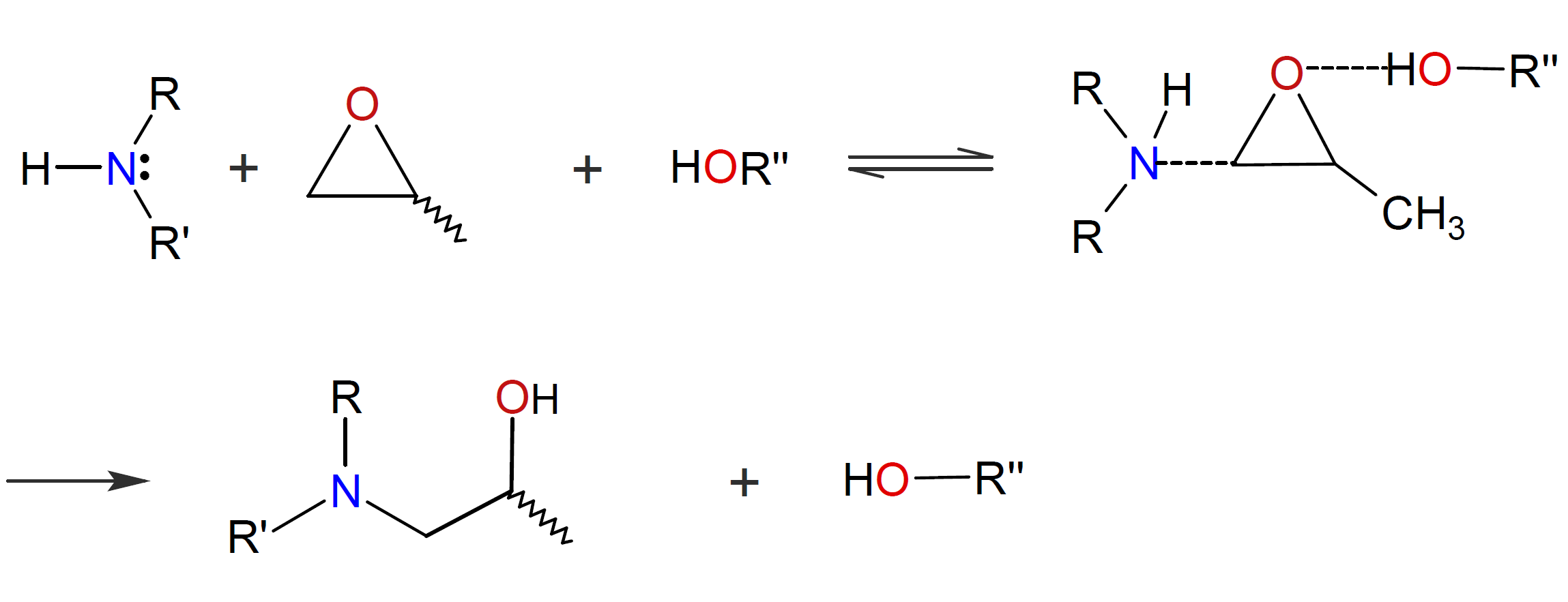
The reaction between amines and epoxy resins is significantly accelerated by proton donors such as water, phenols, or glycols. A mechanism has been proposed in which a hydroxyl hydrogen atom partially protonates the oxygen atom of an oxirane ring, making the neighboring methylene group more susceptible to attack by a nucleophilic amine:1,2
Amine-epoxy thermosets typically have good elevated temperature performance and outstanding chemical resistance. On the downside,
many amine curatives are (highly) toxic, require relative long cure cycles and yield amber colored products.
Oxirane rings may also react with hydroxyl groups (OH) that are present in the polyol accelerators
and are formed when an
oxirane ring reacts with a primary or secondary amine. This addition reaction is known as
etherification, even when the OH group resides on the polymer backbone.1
Furthermore, cyclic ethers can undergo homopolymerization,
particularly at elevated temperatures and in the presence of a
catalyst such as tertiary amines.
2) Thiol-Epoxy Addition Reactions
Base-catalyzed thiol-epoxy additions can be categorized as click reactions, which means they are highly selective, produce no by-products and take place under mild reaction conditions with high yield. They are also much faster than epoxy-amine additions, particularly at low temperatures, and may yield clear products. However, the mechanical and physical properties are typically inferior to those of amine crosslinked epoxies. Furthermore, most thiols have a disagreeable odor which, however, usually disappears after cure.
The base-catalyzed thiol-epoxy addition is assumed to take place as a nucleophilic addition between a thiolate anion and an epoxy group. The reaction is initiated by a sufficiently strong base such as a tertiary amine which undergoes an acid-base proton exchange with a thiol group to produce a thiolate anion which subsequently attacks an oxirane ring to produce an alkoxide ion, which then undergoes a proton exchange with a thiol group.3,4 The thiol-epoxy reaction is strongly autocatalytic because the formed hydroxyl groups facilitate ring-opening of the epoxy group.4,5 The three steps of the thiol-epoxy ring-opening copolymerization are shown in the scheme below.
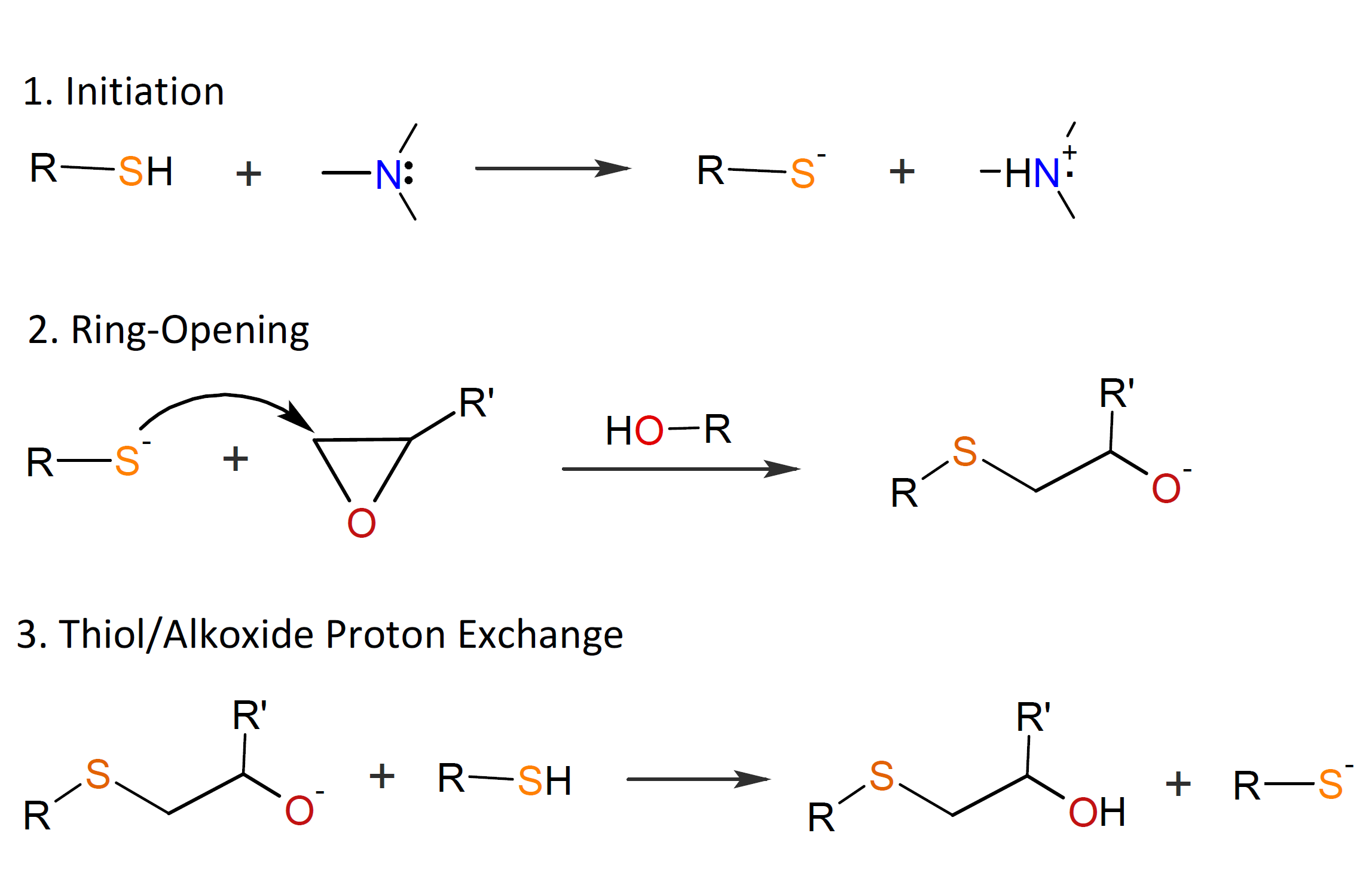
Alternatively, the amine may attack an oxirane ring which produces an alkoxide anion, which then abstracts a proton from a thiol.
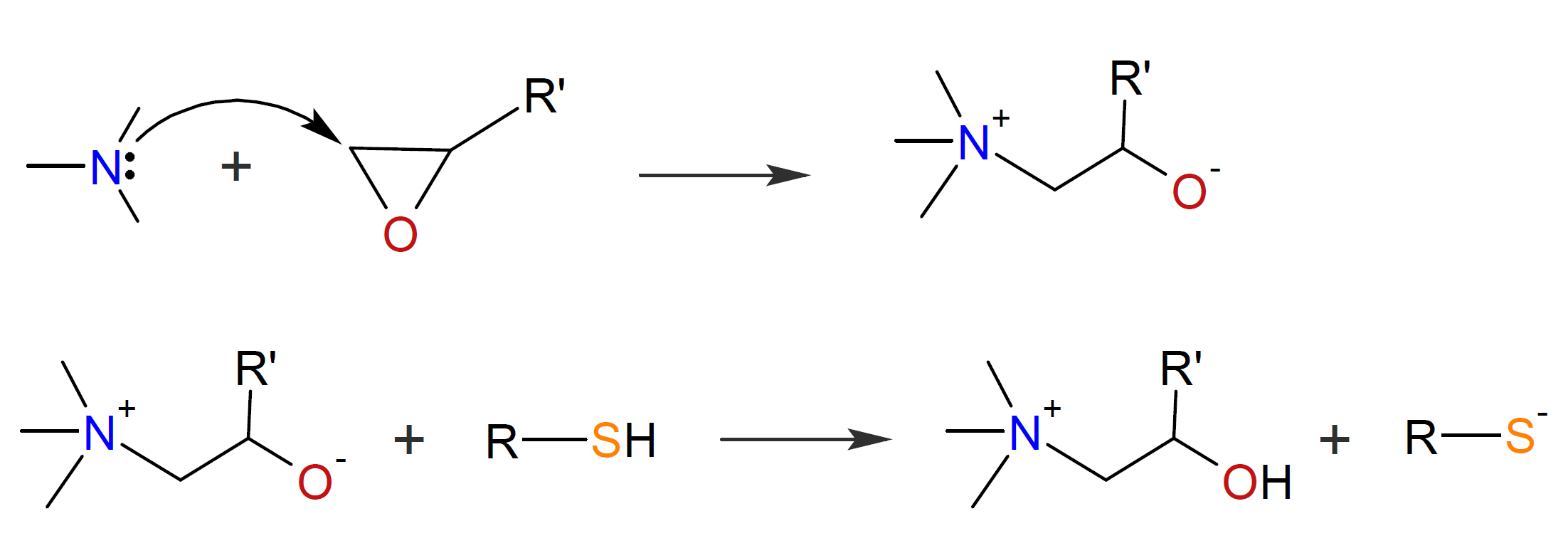
Which of these initiation steps prevails depends on the basicity and nucleophilicity of the tertiary amine, or on the presence of a proton donor acting as a co-catalyst.3,6
3) Alternating Ring-opening Copolymerization (ROCOP)
Cyclic ethers such as oxiranes, oxetanes, and oxolanes can undergo alternating ring-opening copolymerization (ROCOP) with other (cyclic) compounds such as mono-, bi- and tricyclic anhydrides, lactones and carbon dioxide.8-10 These reactions require an initiator and yield polyesters or polycarbonates. Often single site metal complexes are employed which allow for the preparation of well-defined polymer architectures. The polymerization mechanism is commonly described as an anionic ring opening chain polymerization including an initiation, a propagation and a termination step.10 Two examples of polymeric compounds that can be produced by ROCOP are shown in the figure below.
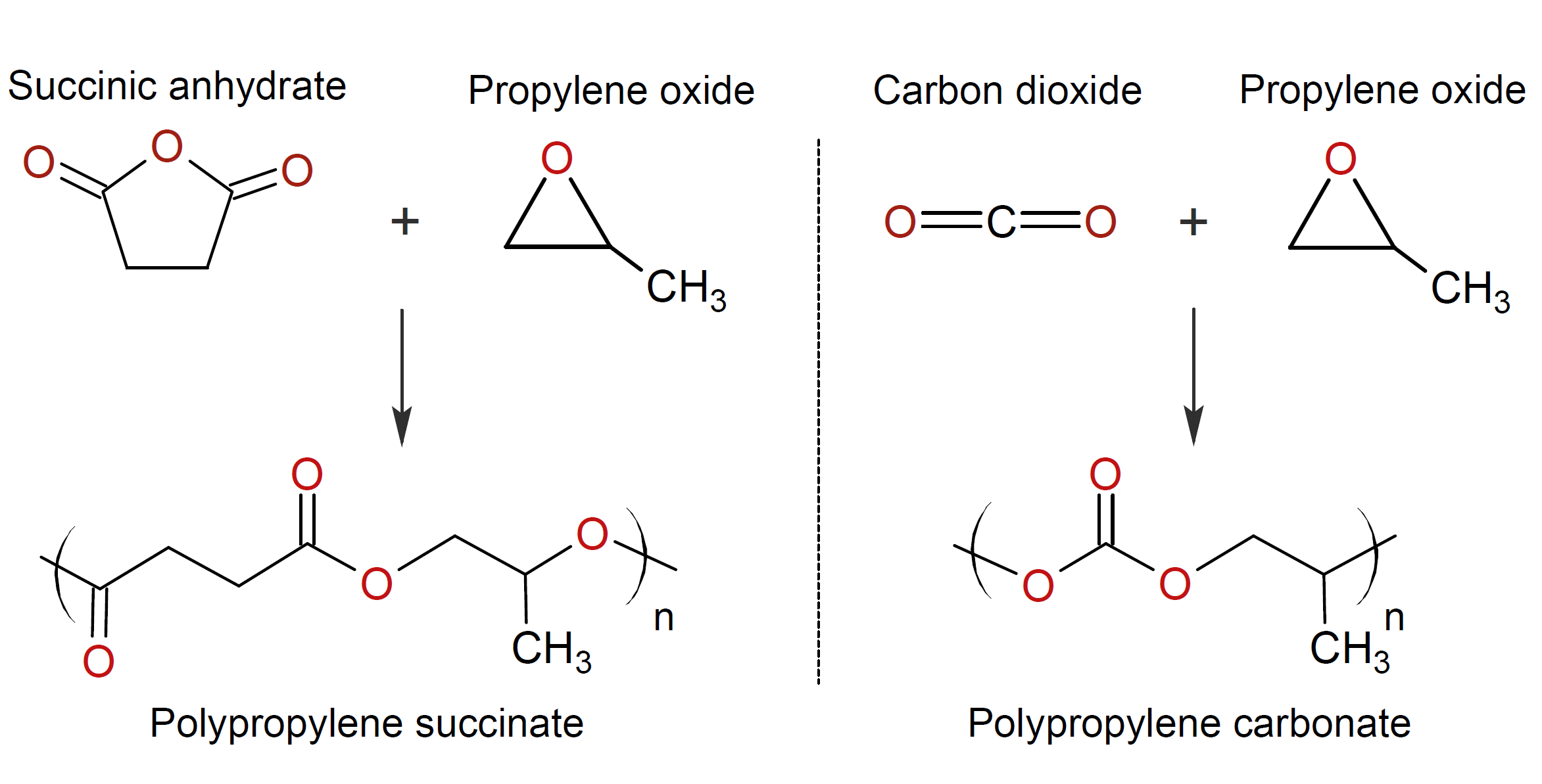
Hundreds of other possible polymer structures can be synthesized with a wide range of properties such as low and high glass transition temperature allowing for tailoring the thermal and mechanical properties to the desired application.
Anhydrides are also frequently employed as crosslinking agents for epoxy resins. These thermosetting systems have in many cases better properties than epoxy resins cured with amines or thiols. For example, they are typically less toxic, have a higher glass transition temperature, show less reaction shrinkage, and are less exothermic.11 On the downside, (catalyzed) epoxy-anhydride systems have a low reactivity and therefore, curing has to be carried out at elevated temperatures. Typically, strong Lewis bases such as tertiary amines are employed to speed up the reaction.12
Notes & References:
- I. A. Saeedi, T. Andritsch and A. S. Vaughan, Polymers, 11, 1271, 1-18 (2019)
- A. Ravve, Principles of Polymer Chemistry, 2nd Ed., Kluwer Academic, New York 2007
- D. Guzman, X. Ramis, X. Ferandez-Francos and A. Serra, RSC Adv., 5, 101623 (2015)
- O. Konuray, X. Fernández-Francos and X. Ramis, Polym. Chem., 8, 5934 (2017)
The effect of alcohols on the reaction was studied by Jin et al.;7 they found that their effect was less important than that of hydroxyl anions produced by the thiol–epoxy addition which strongly facilitate ring-opening of the epoxy group.
An alternative reaction mechanism has been suggested by Konuray et al.,4 which involves the formation of amine-thiol ion pairs that become the main propagating species
- K. Jin, W. H. Heath and J. M. Torkelson, Polymer, 81, 78-78 (2015)
- S. Paul, Y. Zhu, C. Romain, R. Brooks, P.K. Saini and C.K. Williams, Chem. Commun., 51, 6459-6479 (2015)
- J.M. Longo, M.J. Sanford, and G.W. Coates, Chem. Rev., 116, 15167−15197 (2016)
- T. Vidil, F. Tournilhac, S. Musso, A. Robisson, L. Leibler, Prog. Polym. Sci., 62, 126-179 (2016)
- F. Kolar and J. Svitilova, Acta Geodyn. Geomater, Vol. 4, No. 3 (147), 85-92 (2007)
- S. Thomas, C. Sinturel, and R. Thomas, Micro- and Nanostructured Epoxy/Rubber Blends, 1st Ed., Wiley-VCH 2014
21 June, 2020