Cationic Ring Opening Polymerization
Many cyclic compounds that contain heteroatoms in the ring structure are prone to undergo cationic ring-opening polymerization (CROP). Particularly susceptible to CROP are small heterocyclic monomers with large ring strain such as 3-, 4-, and 5-membered rings whereas 7- and 8-membered rings are less reactive due to their much lower ring strain but might be still reactive enough to polymerize,1 because ring-opening leads to greater degrees of freedom of rotation compared to cyclic monomers.
Monomers that are susceptible to CROP typically possess polarized bonds in the ring structure which are represented by Y - X where Y is an atom or a functional group that has a lone electron pair
(Lewis base) such as oxygen, nitrogen or sulfur. These polarized bonds
provide a site for nucleophilic attack by an initiating electrophile
Z+ (Lewis acid or proton). X is often a methylene group
which may become the propagating cationic growth center after
ring-opening.
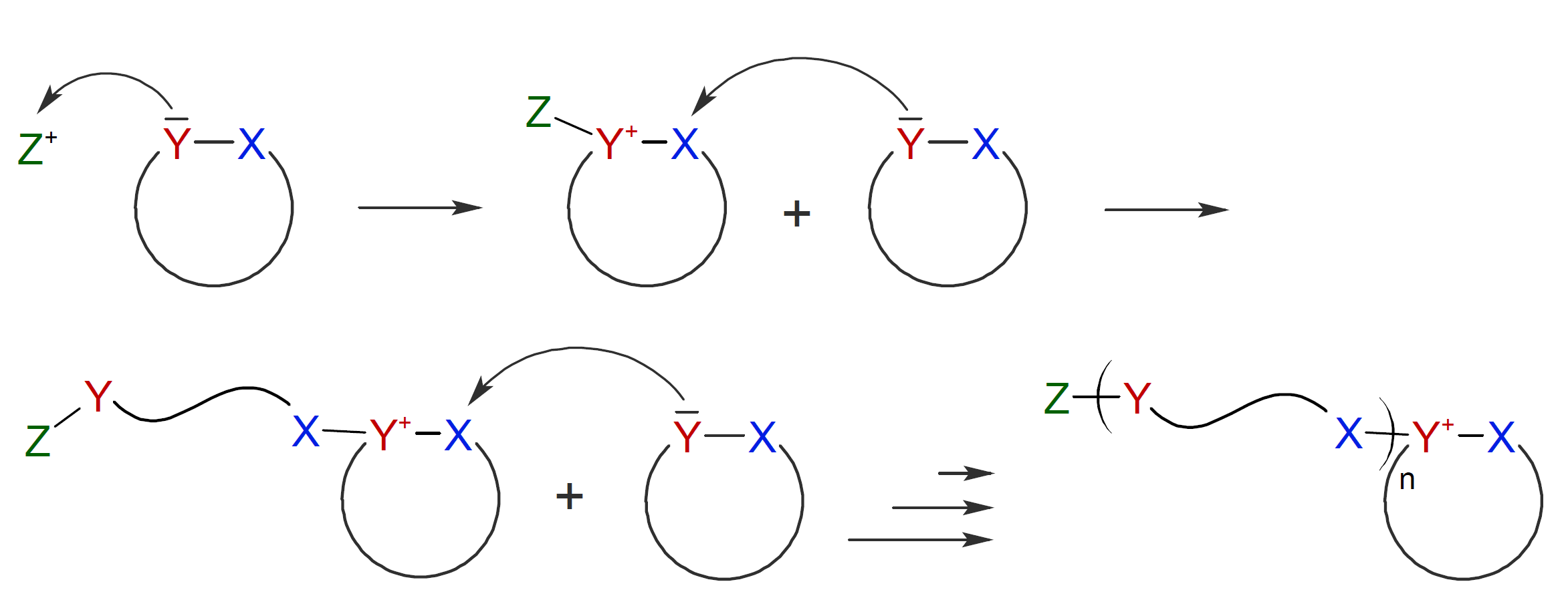
Two CROP mechanisms have been suggested in
the literature,2 both involve a nucleophilic attack of a cyclic
monomer molecule towards the electrophilic initiator. In the first
case, the resulting cyclic onium ion undergoes ring opening when
attacked by the nucleophilic atom Y of another monomer. This
mechanism is known as bimolecular nucleophilic ring-opening:
Alternatively, the activated ring can undergo spontaneous ring-opening resulting in an acyclic cationic species (X+) which than can be attacked by a monomer (monomolecular nucleophilic ring-opening):
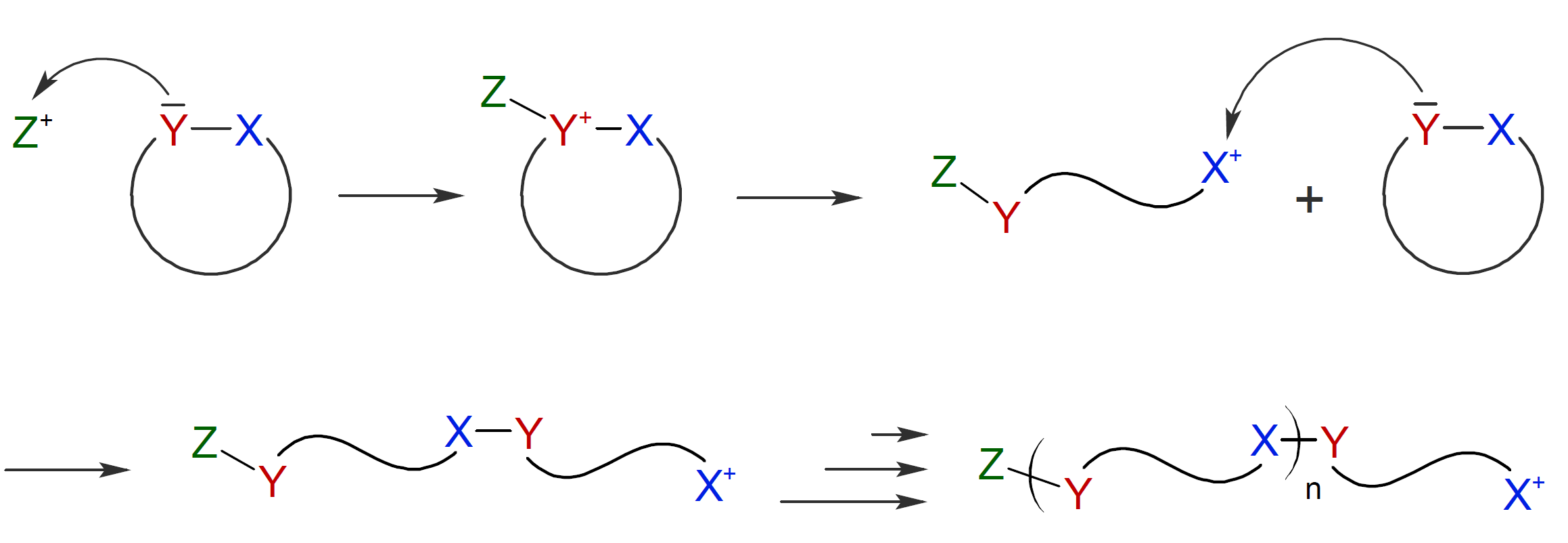
Which of these two mechanisms prevails, will depend on the
stability of the cation. If X+
is sufficiently stabilized by electron donating neighboring groups, a
mono-molecular ring-opening process will be predominant.
It is also
possible that the monomer is activated by the electrophile and
carries the cationic center. In this case, the activated monomer is
the growth center which then adds to a neutral chain end with
simultaneous release of a hydrogen ion (proton):1,2
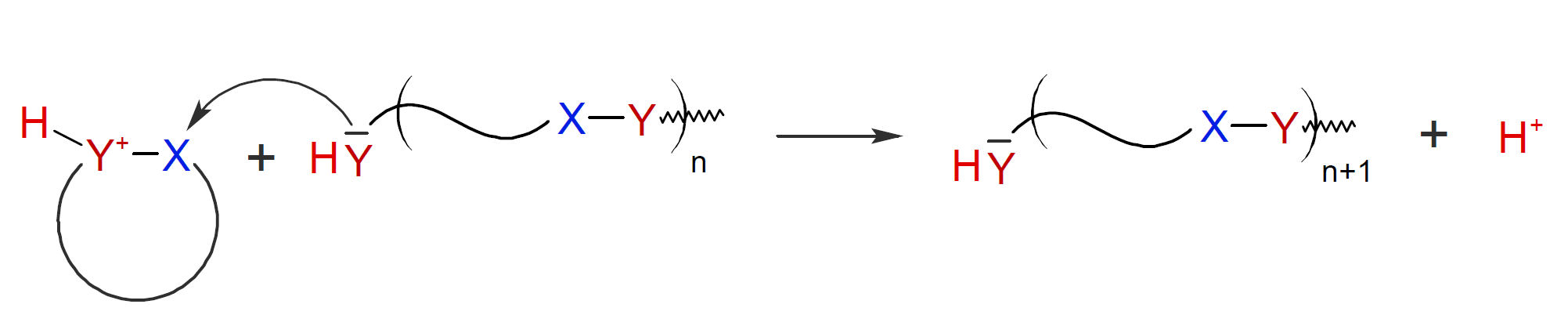
For some CROP systems the mechanisms above may compete with each other.1-3 The propagation reactions may also compete with inter- and intramolecular side reactions including back-biting (a) and ring eliminations (b):
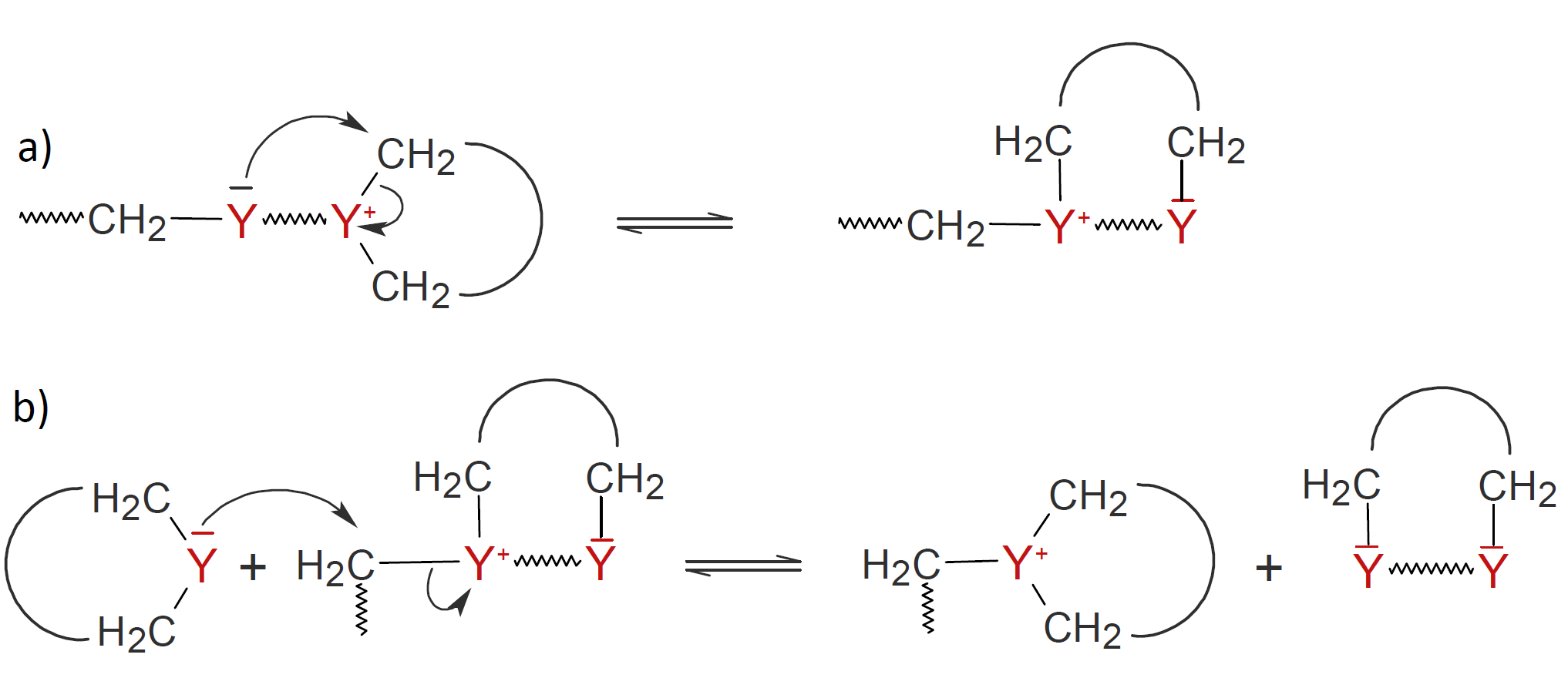
The competition between these side reactions and chain propagation leads to a broader molecular weight distribution. However, in most case, side reactions are less frequent events due to entropic and steric effects. For example, back-biting is discouraged because the addition of a cyclic monomer is less sterically hindered than the attack of a heteroatom of a polymer chain during backbiting.4
In contrast to most other chain growth techniques, ring-opening polymerizations are reversible and reach an equilibrium between polymers and unconsumed cyclic monomers. The extend of the reaction will depend on the type of monomer, the ring-size (ring strain) and the reaction conditions. Monomers with high ring strain such as epoxides, oxetanes and aziridines readily polymerize to high molecular weights with negligible residual monomer whereas many five- and six-membered cyclic compounds which are least strained polymerize to only low molecular weight compounds or are unable to undergo polymerization i.e. a very high concentration of unreacted monomer remains at equilibrium.2,4,5
| Monomer & Polymer | Monomer Structure | Repeat Unit |
Oxiranes (Epoxides) |
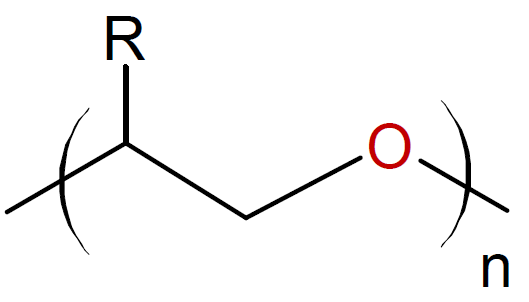 |
|
Oxitanes |
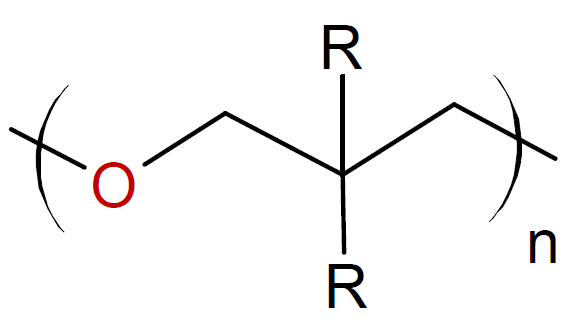 |
|
Caprolactone |
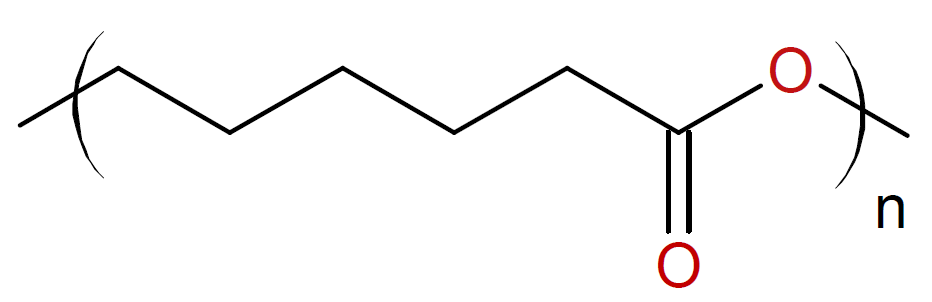 |
|
Trioxane |
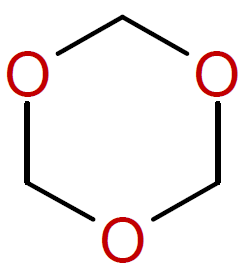 |
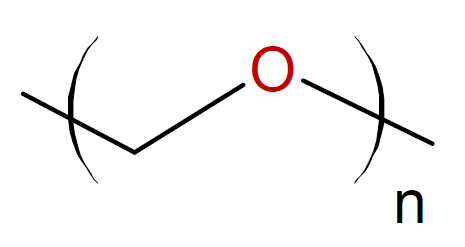 |
2-Oxazoline |
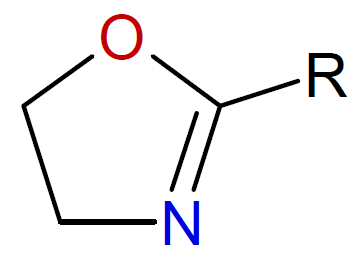 |
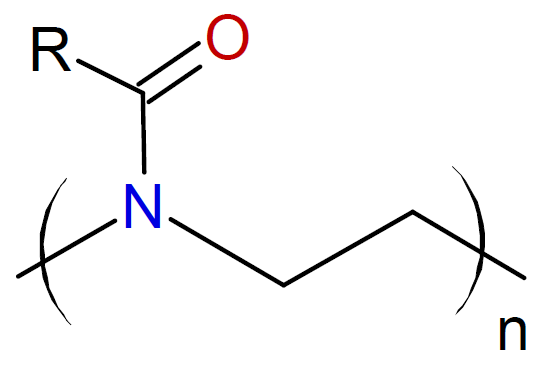 |
Tetrahydrofuran |
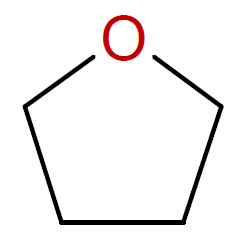 |
Some examples of cyclic monomers that polymerize through cationic ring-opening polymerization include cyclic ethers (oxiranes & oxitanes), lactones, lactams, aziridines, phosphazenes, oxazolines, oxazoles and siloxanes. The ring opening polymerization of simple cyclic monomers has gained commercial importance for only small ring sizes, i.e. three- and four-membered rings such as epoxides, oxetanes and aziridines. The polymerization is typically carried out in bulk or at high monomer concentration to depress side reactions such as back-biting and intermolecular chain transfer to polymer which would decrease the final molecular weight and produce cyclic oligomers. The polymerization of cyclic acetals such as trioxane and dioxolane are more difficult to control because they are more prone to inter- and intramolecular chain transfer than cyclic ethers due to the greater basicity of the oxygen compared to cyclic ethers.6,7
Notes & References:
1O. Nuyken 1 and S.D. Pask, Polymers 5, 361-403 (2013)
2Philippe Dubois,Olivier Coulembier,Jean-Marie Raquez, Handbook of Ring-Opening Polymerization, 2009
3S. Pemczek, J. Polym. Sci. Part A: Polym. Chem., Vol. 38, 1919–1933 (2000)
4Wei-Fang Su, Ring-Opening Polymerization. In: Principles of Polymer Design and Synthesis. Lecture Notes in Chemistry, Vol 82. Springer, Berlin 2013
5The ring strain of 3-, 4-, and 5-membered lactones is about 116, 111, and 27.5 kJ/mol, respectively.8
6Note, the six-membered trioxane readily undergoes polymerization whereas most five- and six-membered cyclic ethers do not polymerize due to the low ring strain.4
7The polymerization of trioxolane when catalyzed with Lewis acids and water proceeds rapidly in the melt at 60 to 70°C.8 However, the reaction is hard to control due ot the high exothermicity of the reaction. The resulting polyoxymethylene is an important engineering polymer.
8 A. Ravve, Principles of Polymer Chemistry, 2nd Edition, Kluwer Academic, New York 2000
24 May, 2020