Anionic Ring Opening Polymerization
Many cyclic compounds that contain heteroatoms in the ring structure can undergo anionic ring-opening polymerization (AROP). Particularly susceptible to AROP are small heterocyclic monomers with large ring strain such as 3-, 4-, and 5-membered rings whereas 7- and 8-membered rings are less reactive due to their much lower ring strain but might be still reactive enough to polymerize,1,6 because ring-opening leads to greater degrees of freedom of rotation compared to cyclic monomers.
Typical monomers that can undergo anionic ring-opening polymerization possess strongly polarized bonds such as ester, carbonate, amide, urethane and phosphate, which polymerize to polyester, polycarbonate, polyamide, polyurethane and polyphosphate, respectively. Cyclic monomers with less electrophilic groups can also be polymerized if the ring strain is large as it is the case with three-membered rings with ether, amine and thioether functionality. Some examples of anionically polymerizable heterocycles and their corresponding polymers are listed in the table below.
| Monomer / Polymer | Monomer Structure | Repeat Unit |
Oxiranes (Epoxides)
/ |
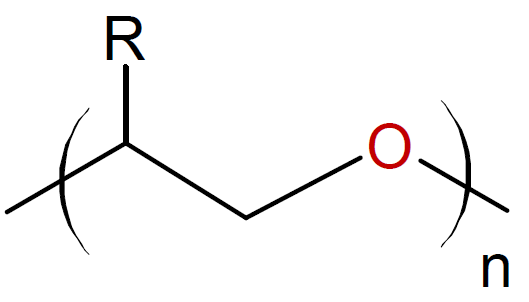 |
|
Caprolactone / |
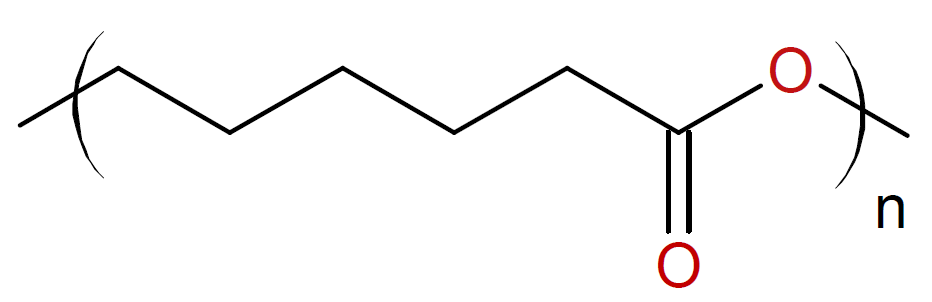 |
|
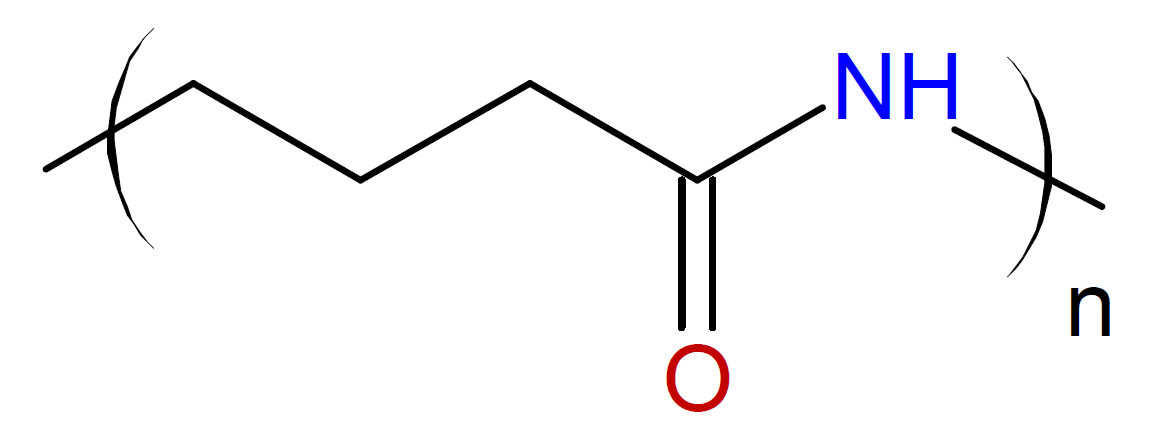
Caprolactam / |
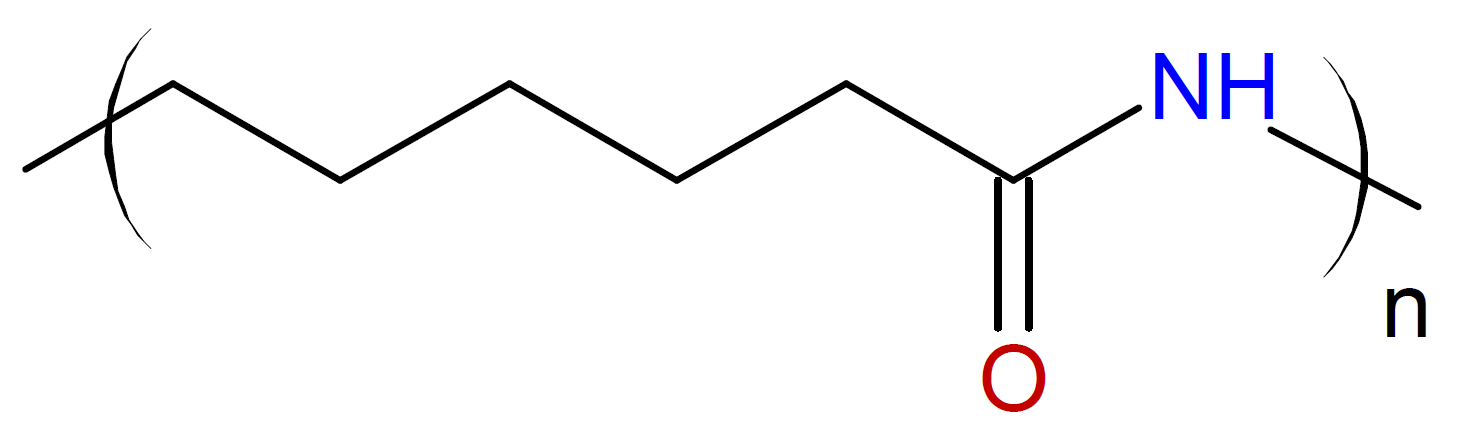 |
|
2-Pyrrolidone / |
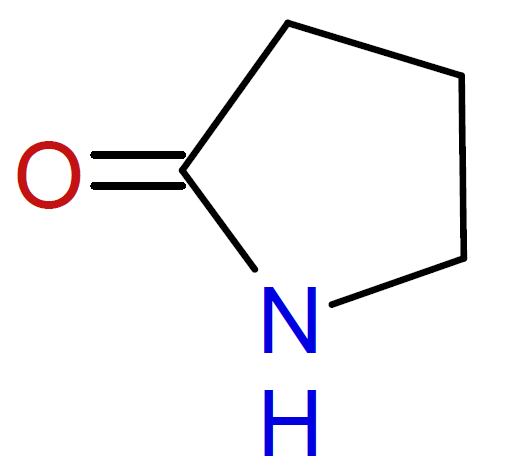 |
 |
AROP can be initiated by a large number of anionic initiators including carbanions, alcoholates, silanoates, carboxylates, thiolates, alkoxides, and tertiary amines. The mechanism of AROP is shown in the scheme below:
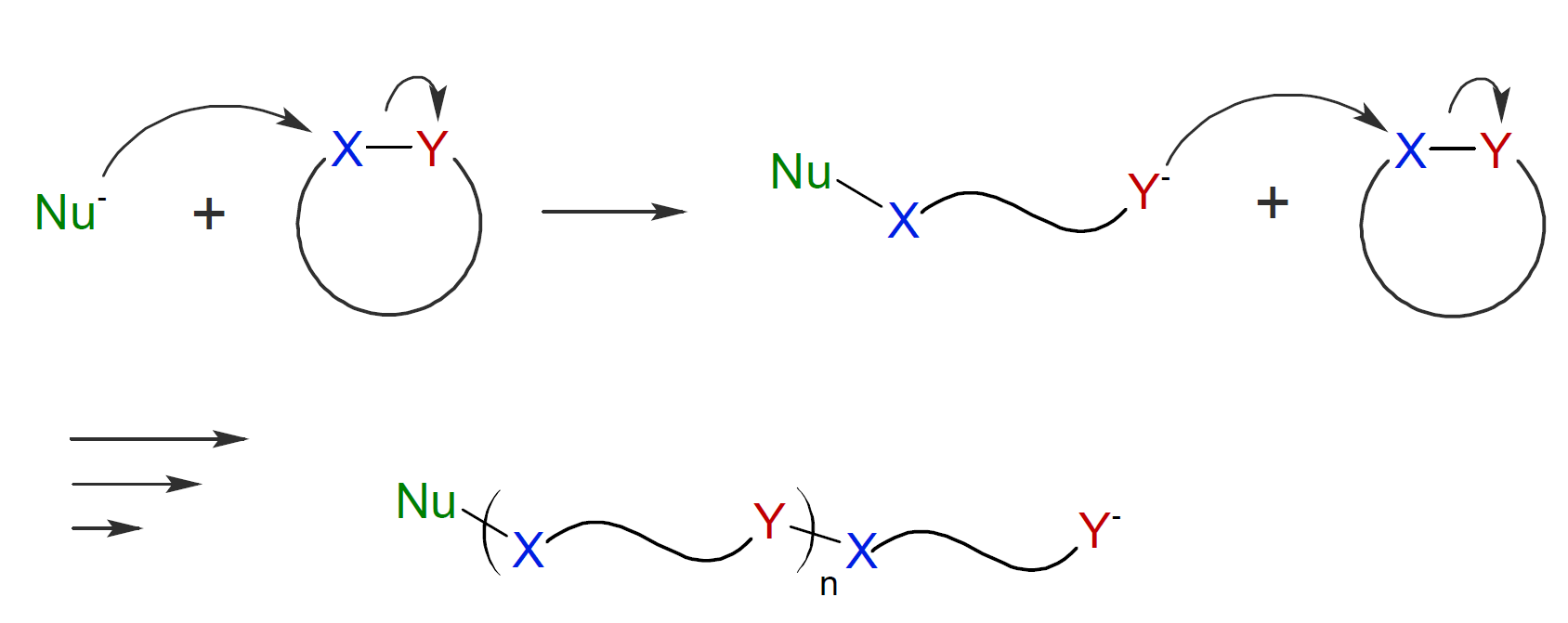
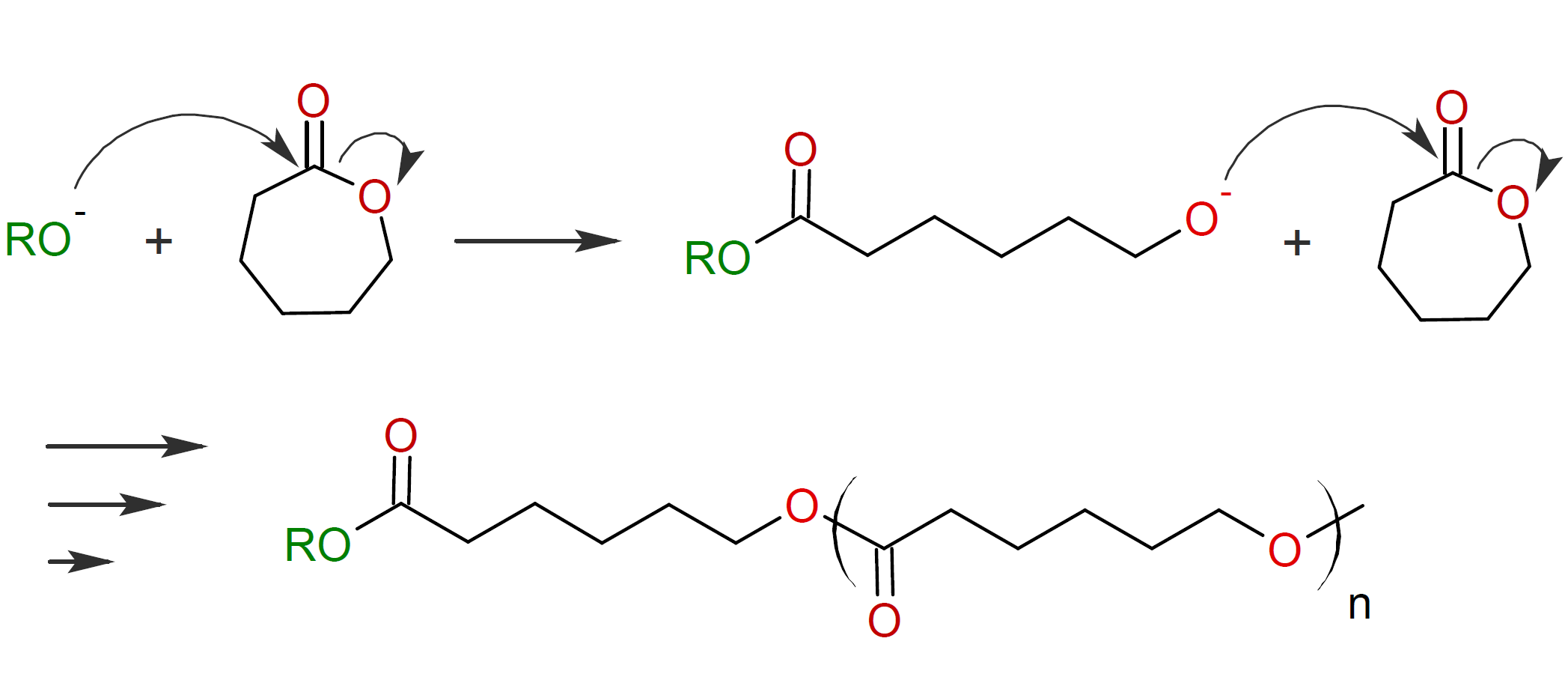
Monomers that are susceptible to AROP typically possess polarized bonds in the ring structure which are represented by Y - X where Y is an atom or a functional group that has a lone electron pair (Lewis base) such as oxygen, nitrogen or sulfur. These polarized bonds provide a site for nucleophilic attack by an initiating nucleophile Nu- (Lewis base). X is often a methylene group or a carbonyl carbon which forms a bond to the nucleophile. A commercially important example of AROP, is the polymerization of caprolactone which readily polymerizes in the presence of metal alkoxide or alkyl alkoxide activators:1
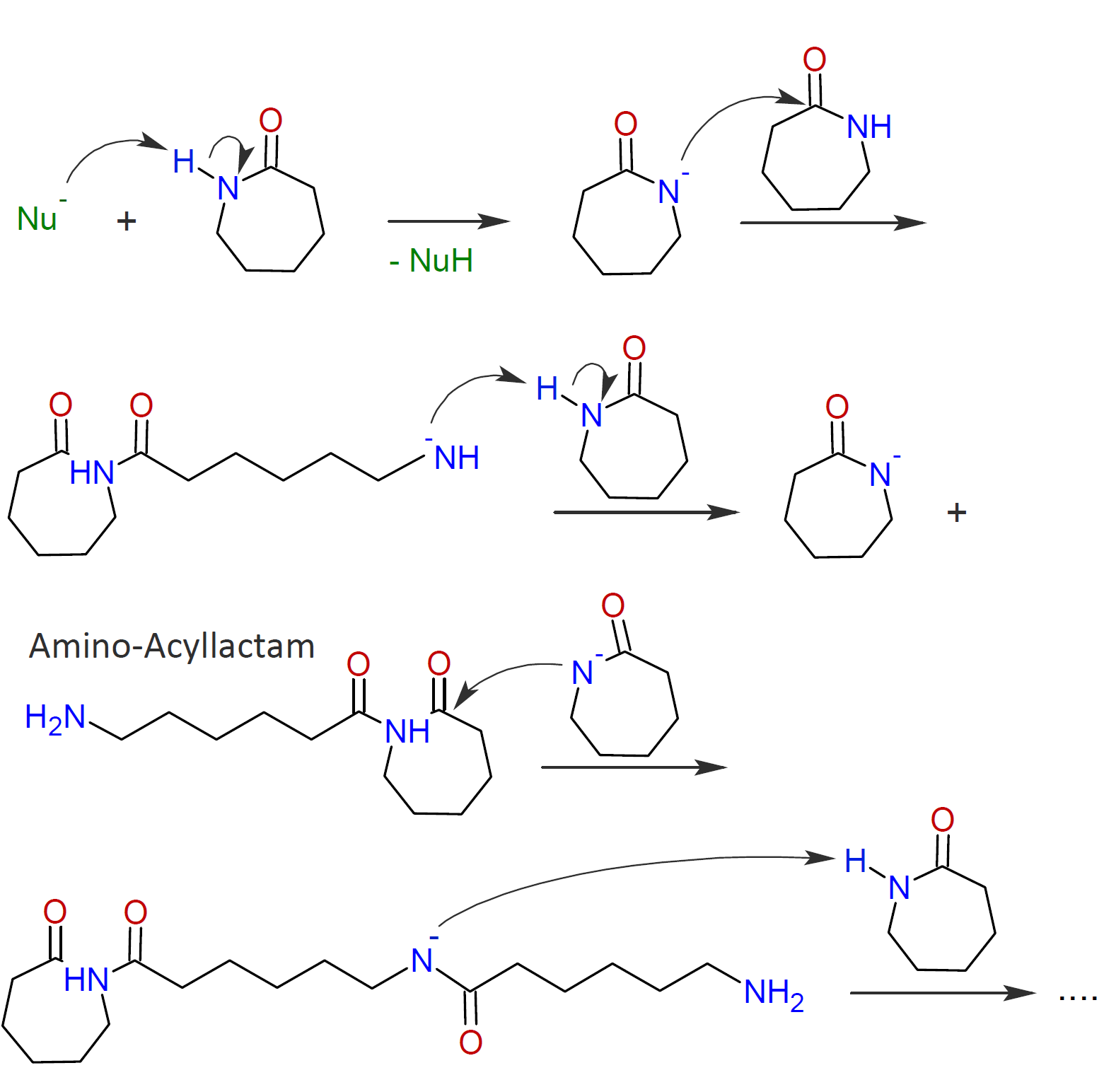
The ring-opening polymerization of caprolactam is more complex; the proposed mechanism involves the abstraction of an acidic H-N proton from the caprolactam in the presence of a strong base. The activated monomer anion then attacks another lactam molecule to induce ring-opening. In a third step, the formed amide anion abstracts a H-N proton from another lactam molecule which produces an amino-acyllactam and another activated caprolactam anion which in turn can react with the N-acyllactam and so on:2,3
Another class of polymers of industrial importance are polyethers which can be produced by anionic ring-opening polymerization of cyclic ethers. In the case of asymmetrical rings such as propylene oxide, two different propagating species can be produced because the ring possesses two dissimilar reactive heteroatom-carbon bonds on the ring:
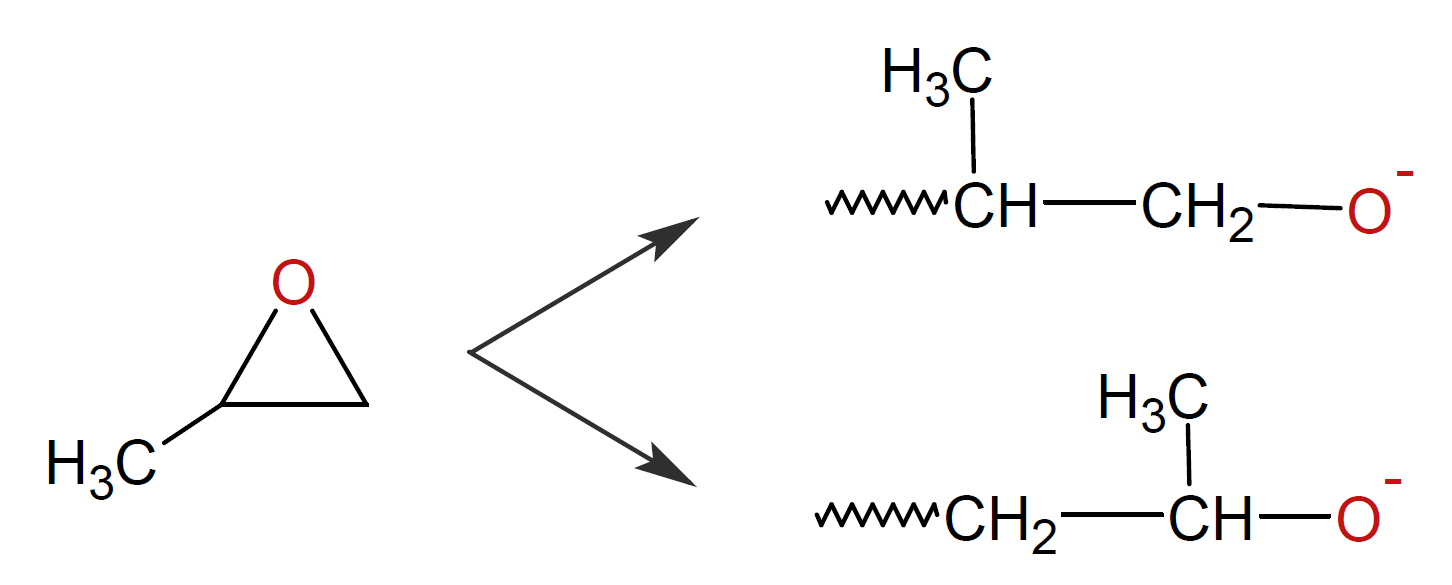
However, due to strong steric hindrance, the unsubstituted carbon is preferentially attacked leading to a predominately head-to-tail placement.4
Steric hindrance also reduces the rate of propagation. For this reason, propylene oxide polymerizes more slowly than does ethylene oxide.
Anionic ROP of cyclic ethers such as epoxides offers several advantages over CROP. For example, AROP of ethylene oxide (EO) proceeds with little macrocycle formation, whereas cationic ROP proceeds with substantial amounts of cyclic oligomers due to the high nucleophilicity of the oxygen atoms in the polymer chain that readily react with the cationic chain-ends.2,5 Furthermore, anionic polymerizations of epoxides can proceed as living polymerization,4 which yields polyethers with narrow polydispersity and well-defined structure. However, AROP is often accompanied by chain transfer to monomer which leads to low molecular weight as it is the case with propylene oxide.4
Like ionic chain growth, ionic ring-opening polymerization shows analogous solvation effects of solvent molecules and counterions, i.e. the propagation center can be different species such as contact ion pairs or solvent-separated ions. In many cases, the propagating anionic chain end forms ion pairs or covalent bonds.2 The different states of solvation and aggregation of the propagating carbanions in polar and nonpolar solvents significantly affects the rate of polymerizations and polymerization kinetic.
Notes & References:
1O. Nuyken 1 and S.D. Pask, Polymers 5, 361-403 (2013)
2Philippe Dubois,Olivier Coulembier,Jean-Marie Raquez, Handbook of Ring-Opening Polymerization, 2009
3 A. Ravve, Principles of Polymer Chemistry, 2nd Edition, Kluwer Academic, New York 2000
4Wei-Fang Su, Ring-Opening Polymerization. In: Principles of Polymer Design and Synthesis. Lecture Notes in Chemistry, Vol 82. Springer, Berlin 2013
5 J.G. Bots, L. van der Does, A. Bantjes, J. Boersma, Makromol. Chem., 188, 1665 (1987)
6The ring strain of 3-, 4-, and 5-membered lactones is about 116, 111, and 27.5 kJ/mol, respectively.3
30 May, 2020