Ring Opening Metathesis Polymerization (ROMP)
Ring-opening metathesis polymerization (ROMP) is a type of olefin metathesis chain-growth reaction.1 The method is extensively used to prepare stereoregular and monodisperse linear polymers and co-polymers formed from unsaturated cyclic olefins such as cyclobutene, cyclopentene as well as from bi- and tri-cyclic unsaturated rings such as bicycloheptene (norbornene) and dicyckopentadiene (DCPD).
The main driving force for any ring-opening polymerization is the release of ring strain energy which is inherent to 3, 4, 5 and 7 to 11 membered cycloalkenes whereas 6-membered rings have negligible ring strain energy.2
ROMP can be used to synthesize completely linear and stereoregular polymers and copolymers. The stereochemistry depends on the reaction conditions and the type of catalyst. A common commercial ROMP polymerization uses a RuCl3/HCl in combination with a promoting agent such as EtOH or PhOH. However, these industrial catalysts are heterogeneous and ill-defined systems whereas academic research has led to the development of well-defined molecular single-component catalysts.3 Many of these are metal-alkylidene (carbene) complexes, consisting of a halide, oxide or oxo-chloride of a transition metal such as W, Mo, Rh, or Ru and an alkylating agent (Lewis acid) such as tetraalkyltin R4Sn or alkylaluminum dichloride RAlCl2, which produce metal carbenes in situ. A very popular catalyst is the so-called Grubbs initiator which have significantly improved chemical stability to oxygen and moisture.
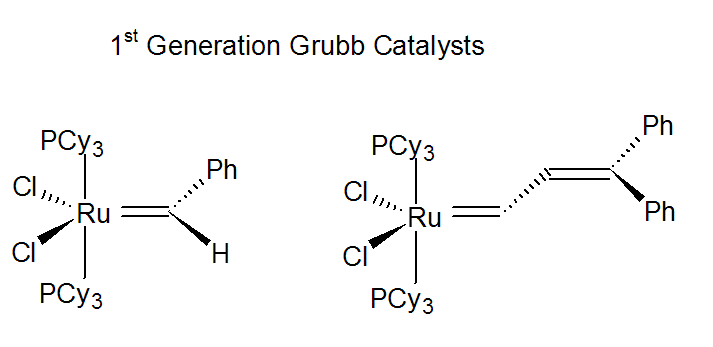
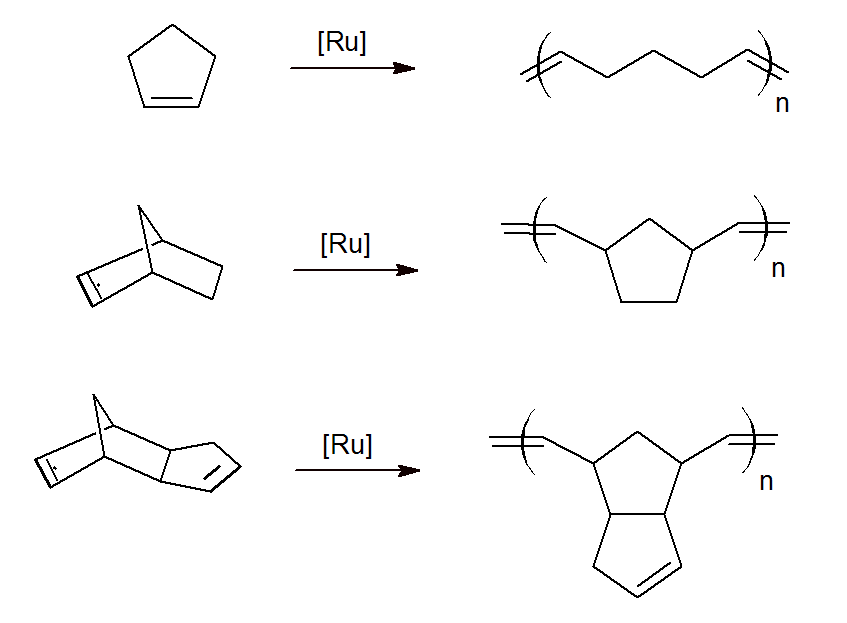
Ch is a cyclohexyl and Ph is a phenyl substituent. The reaction sequence starts with ring opening in the presence of a [Ru] catalyst forming a linear double bond with the transition metal with a terminal double bond as well. The new carbene then reacts with the double bond of another monomer, thus, creating a chain growth reaction. This process proceeds at low temperatures of about 25 - 100 °C when using a Grubb catalysts.
A number of cycloalkene and bicycloalkenes have been polymerized to high-molecular weight polymers via ROMP including cyclopentene, norbornene, and endo-dicyclopentadiene. The later two have found many commercial applications.
References & Notes
The olefin metathesis was invented by Chauvin, Grubbs and Schrock who were awarded the Nobel prize in 2005 for their work.
The highest strain energy is encountered in 3- and 4-membered rings. These monmers rapidly polymerize in the presence of a transition metal carbenes (alkylidene catalyst).
-
Christoph Janiak, Paul G. Lassahn, Macromol. Rapid Commun., 22, No. 7, pp. 479 - 492 (2001)
-
J. Cui, J.-X. Yang, Y.-G. Li, and Y.-S. Li, Polymers, 7, pp. 1389-1409 (2015)
-
M.V. Bermeshev, B.A. Bulgakov, A.M. Genaev, J.V. Kostina, G.N. Bondarenko, and E.Sh. Finkelshtein, Macromolecules, 47 (16), pp. 5470-5483 (2014)