Cationic Photoinitiators
Cationic photoinduced polymerization was first discovered by Crivello and Lam in the mid-1970s when they observed that UV irradiated onium salts such as triphenylsulfonium or diaryliodonium generate strong acids, which were able to initiate cationic polymerization.1-3 They also observed that the initiating moities are chemically stable for a very long time. As a consequence, polymerization, once initiated, continues even in the absence of light (dark cure). Thus, monomers and/or oligomers can be cured with a much lower UV dose. Cationic polymerization also allows for tighter control of molecular weight compared to free-radical photopolymerization. Furthermore, the propagation step is not inhibited by oxygen which is a major problem of radical induced photo-polymerization.4 On the downside, chain growth and crosslink reactions are easily inhibited by trace amounts of water and basic chemicals such as amines, urethanes, as well as by basic pigments and fillers which severely limits the choice of raw materials. Another major drawback is the low solubility of the cationic photoinitiators in nonpolar monomers and oligomers which limits the types of monomers that can be polymerized by this method.4,5
Common cationic photoinitiators are onium salts such as triphenylsulfonium salts1, diazonium salts2, diaryliodonium salts2 as well as ferrocenium salts4 and various other metallocene compounds. The protonic acid or Lewis acid formed of these onium salts are relative stable and have little tendency to undergo chain termination reactions, and thus are very efficient photoinitiators.
The efficiency of the onium salts depends to a large extend on their solubility in the resin, which in turn, depends on the polarity and surface charge. In general, the solubility increases with increasing size of the anion because the charge is dissipated over a larger surface area of the anion which lowers the hydrophilicity. Thus, the solubility and reactivity in nonionic resins increases in the order BF4- < PF6- < AsF6- < SbF6-.6 For this reason, the antimony salts are used more often as cationic photoinitiators than the other salts. Besides solubility, spectroscopic properties such as range of light absorption and bond cleavage efficiency affect the rate of initiation.
The mechanism is quite complex, and a simplified schematic representation is depicted below for a triarylsulfonium salt (Ar3S+ MtXn-).7 When exposed to UV radiation the initiator produces a strong Lewis / Broensted acid which then initiates a ring-opening polymerization of cyclic ether resins:1-3
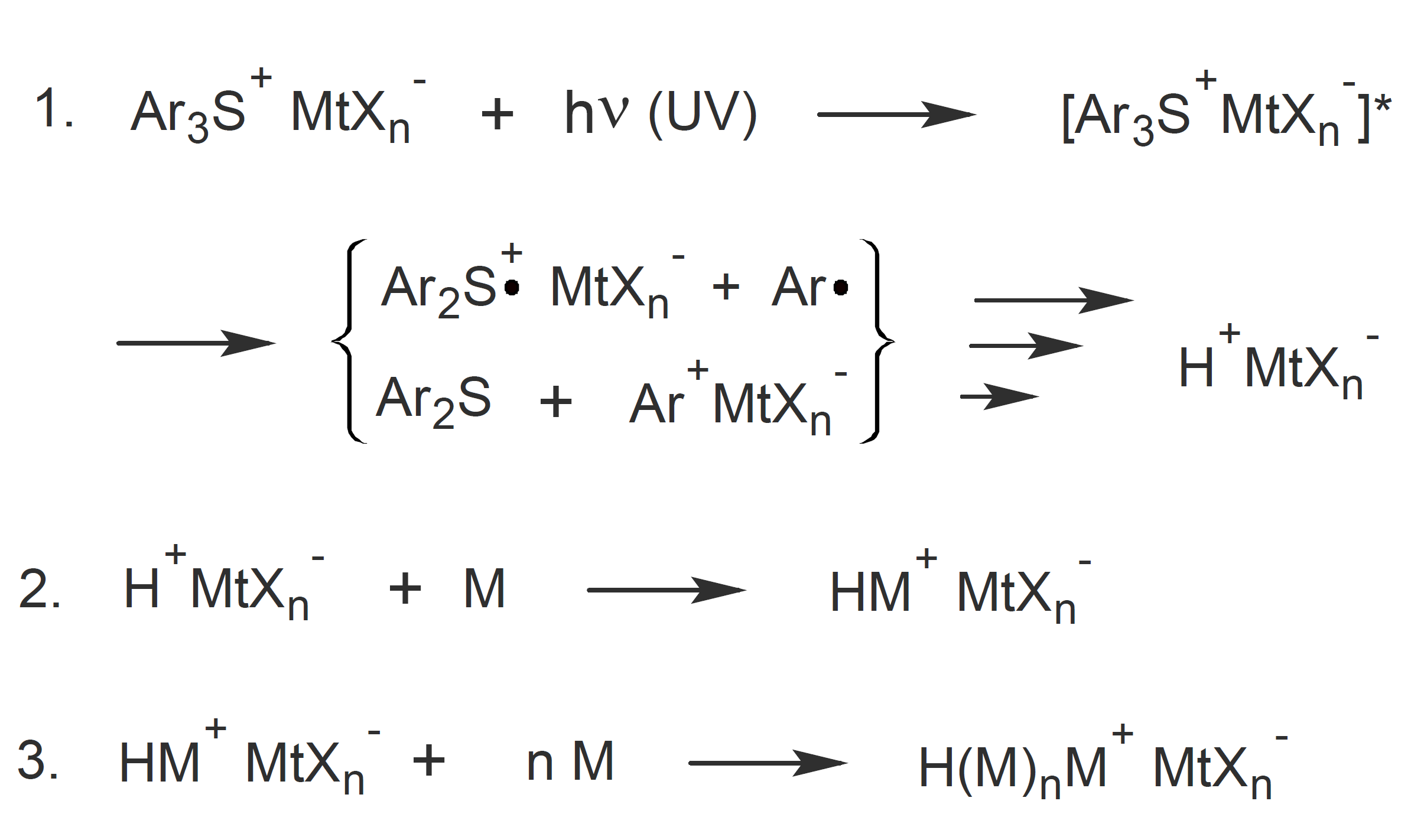
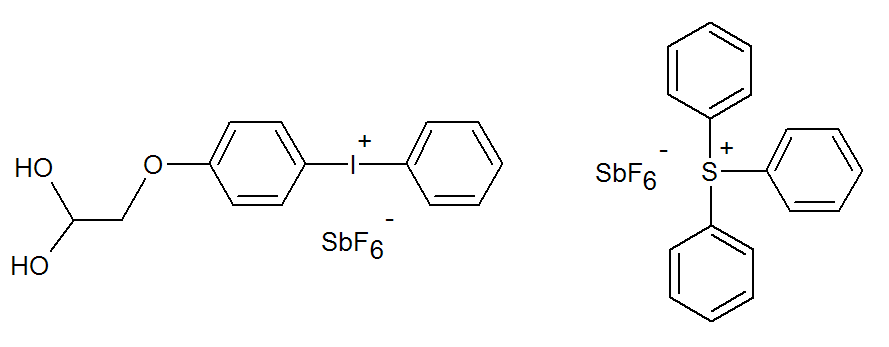
where HMtXn is a Lewis acid such as HBF4, HPF6, HAsF6, HSbF6, Y is a hydrogen donor (solvent or monomer) and M is a monomer. The structure of two very common onium salts, namely diphenyliodonium and triphenylsulfonium salt, is shown below.
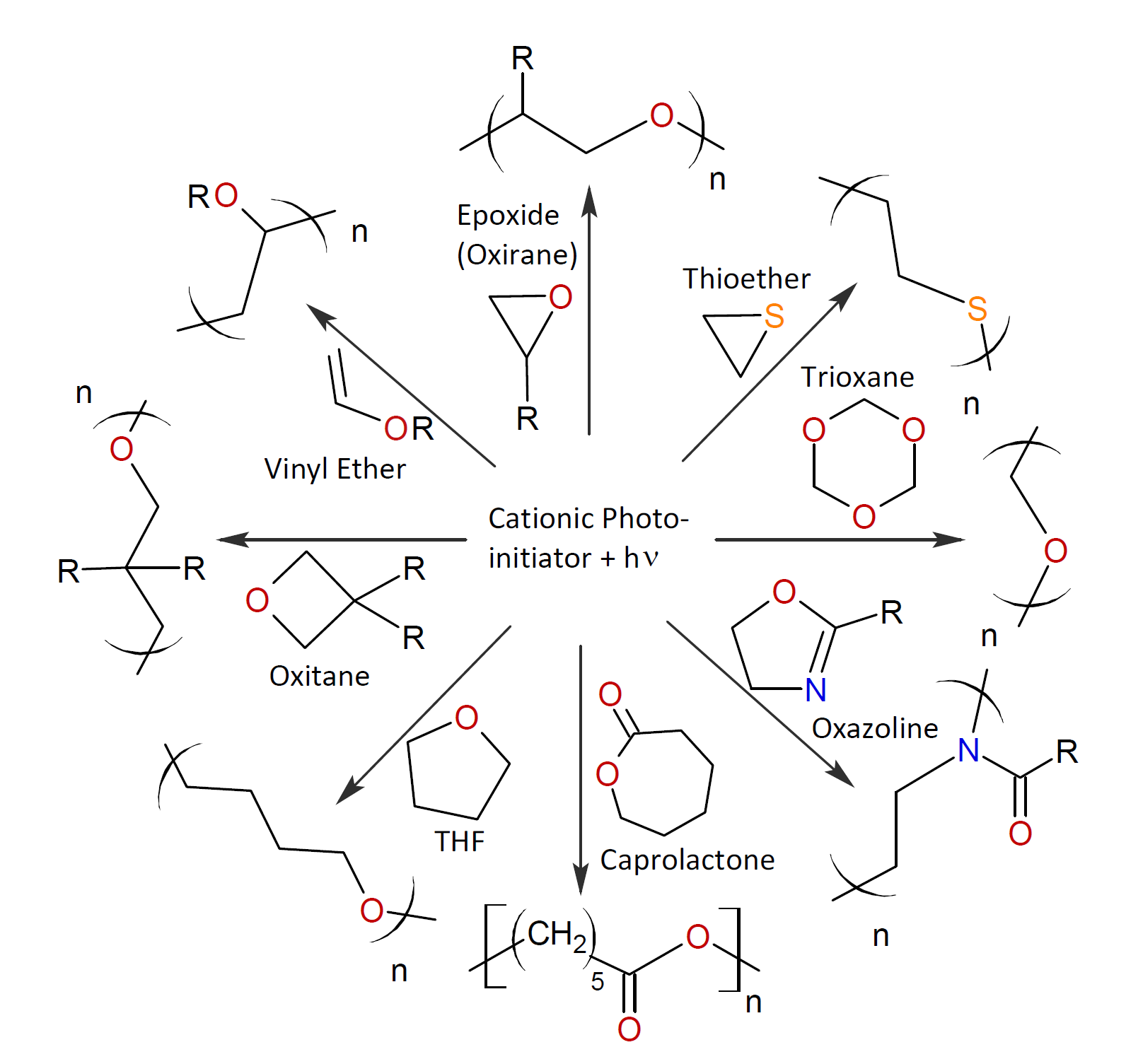
Typical monomers of cationic photopolymerizable compositions are cycloaliphatic epoxides such as 3,4-epoxy cyclohexyl methyl-3,4-epoxy cyclohexane carboxylate (ECC), diglycidyl-1,2-cyclohexanedicarboxylate, 3-ethyl-3-hydroxymethyl oxetane and trimethylolpropane oxetane (TMPO) or epoxy-acrylates such as glycidyl methacrylate and 3,4-epoxycyclohexyl methacrylate. Besides cyclic ethers several other heterocyclic and vinyl compounds are also photopolymerizable including vinyl ethers, caprolactone, oxazoles and cyclic thioethers. Some examples of polymers that can be produced by cationic photopolymerization are shown below.8
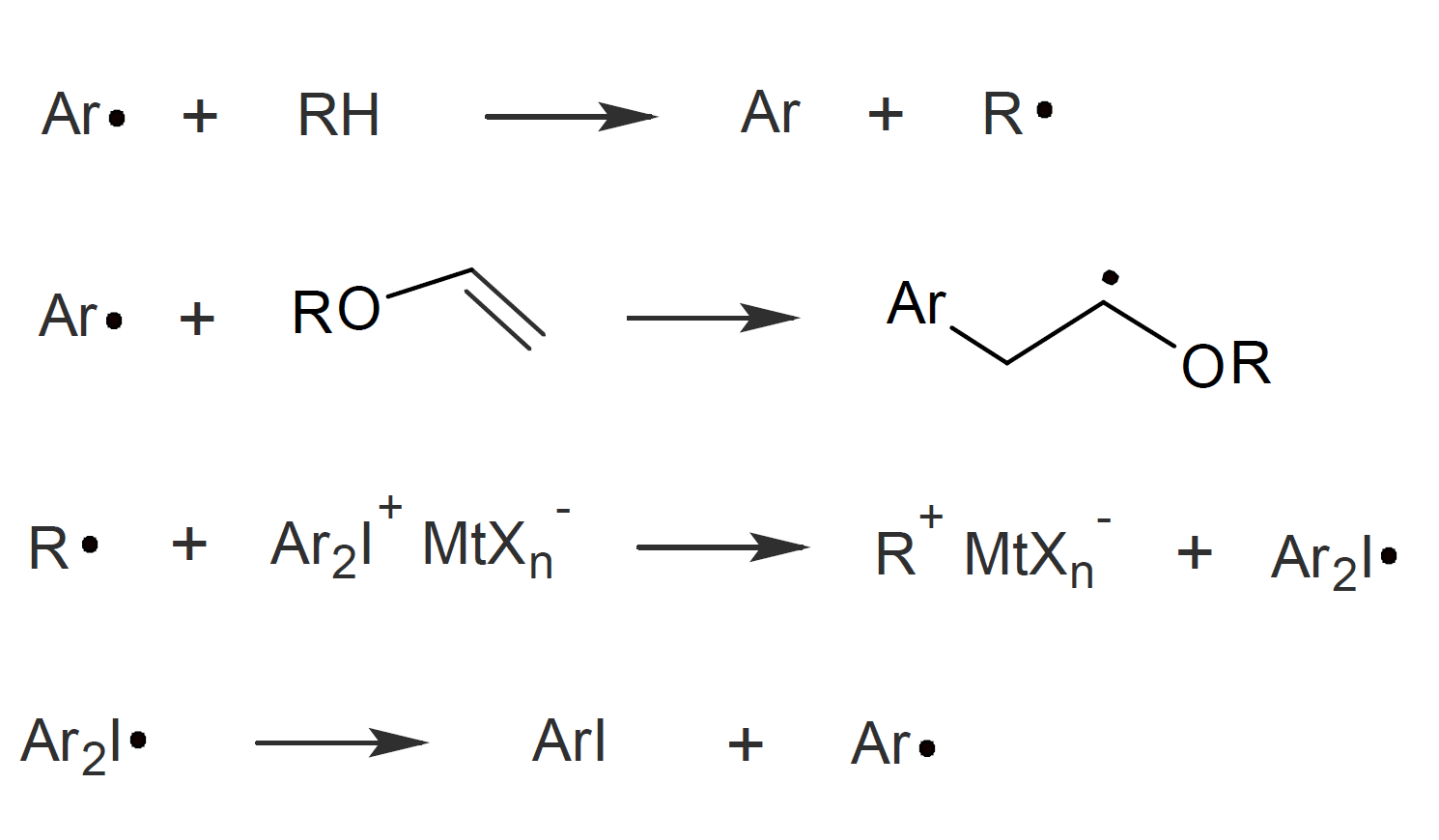
It should be noted that photolysis of the onium salts also produces free radicals. In many cases, these radicals are of little consequence for cationic photopolymerization.5 However, Crivello and Sangermano et al. have shown that the presence of radical species can increase the rate of a cationic ring-opening polymerization.5,8 They suggested that the free radicals can react with the monomers in two different ways; the aryl free radicals can either abstract a hydrogen from a monomer to give a secondary radical or, alternatively, if the monomer bears a vinyl group, the aryl radical can add to the double bond and initiate chain growth. The two radicals can also be oxidized by the onium salt which generates the corresponding cations and a diaryliodine free radical which then initiate cationic polymerization by both inter- and intramolecular attack of a cyclic ether group (epoxide).5
A major drawback of cationic photoinitiation is that the major absorption band of the onium salts lies in the deep UV region and does not overlap with the emission band of visible light. To shift the photoinitiator systems light absorption towards longer wavelengths, the onium salts are sometimes paired with a free radical photoinitiator such as camphorquinone (CQ) together with a hydrogen donor (RH) which acts as a visible light sensitizer.6-10 In the first step, CQ undergoes excitation on irradiation to generate the excited singlet state that then rapidly undergoes intersystem crossing to the excited triplet state. The excited triplet carbonyl readily abstracts a hydrogen atom from the donor (RH) which produces a free radical.
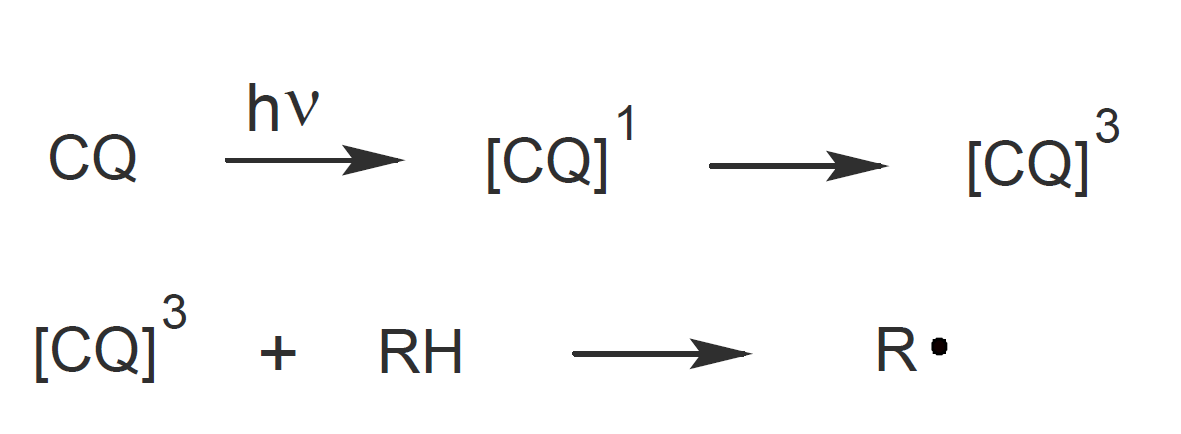
In a third step, the radical is oxidized by the onium salt resulting in cationic species which then initiate cationic polymerization, as shown above.
References & Notes
J.V. Crivello and J.L. Lee, Polymer Journal, Vol. 17, No. 1, pp 73-83 (1985)
-
J.V. Crivello and J.H.W. Lam, ACS Symposium, American Chemical Society, Washington (1979)
-
J.V. Crivello and J.H.W. Lam, J. Polym. Sci., Polym. Symp., 56, 383 (1976)
-
M. Sangermano, I. Roppolo, and A. Chiappone, Polymers, 10, 136 (2018)
-
J.V. Crivello, Designed Monomers and Polymers, Vol. 5, No. 2, 3, pp. 141–154 (2002)
-
M. Sangermano, Pure Appl. Chem., Vol. 84, No. 10, pp. 2089–2101 (2012)
-
A. Vitale, M. Sangermano, R. Bongiovanni, P. Burtscher, N. Moszner, Materials 7, 554-562 (2014)
-
M. Sangermano, N. Razza, J.V. Crivello, Macrom. Mat. Engin., 299 (7), 775-793 (2014)
-
J. V. Crivello, Advances in Polymer Science 62, 1-48 (2005)
-
Aliphatic amines are ofteny used as hydrogen donors. However, their high basicity terminates cationic chain growth. For this reason, hydrogen donors with lower basicity such as ethyl 4-(dimethylamino)benzoate (EMBO) are typically employed.
Revised April 30, 2020