Click Chemistry
The term "Click Chemistry" was introduced by Barry Sharpless and coworkers in 2001 to describe highly efficient and selective reactions that are mainly based on carbon-hetero bond formations. The term "Click" suggests that the molecules are as easily joined together as mechanical snap fasteners. Sharpless et al. described these reagents as being "spring-loaded for a single trajectory." More specifically, the reactions have to be thermodynamically very favorable to "click" which typically requires an enthalpic driving force of at least 20 kcal/mol.1 Such reactions often proceed rapidly under mild conditions and tend to produce a single stereospecific product with high yield.
A click reaction has to meet several other requirements to be useful from the practical viewpoint. For example, a click reaction should be versatile, highly reliable, and applicable to small-scale and large-scale production and the reagents should be readily available. The general definition of click chemistry was given by Sharpless an coworkers as follows:1,2
- modular and wide in scope,
- simple reaction conditions,
- readily available building blocks,
- high reaction rates
- robust reactions with very high yield,
- no or only harmless byproducts,
- simple purification by nonchromatographic methods,
- stereospecific but not necessarily enantioselective,
- oxygen and water insensitive,
- no or only benign organic solvents
Ideally, the starting materials should be inexpensive, non toxic, stable, and of low or inoffensive odor. To minimize cost, the reaction should proceed in bulk, water or in an environmentally friendly solvent and purification or product isolation, if necessary, should be achieved by simple and effective processes such as crystallization or distillation. Furthermore, the final product(s) should be stable under normal storage conditions.
| Click Reactions Employed in Polymer Synthesis and Modifications |
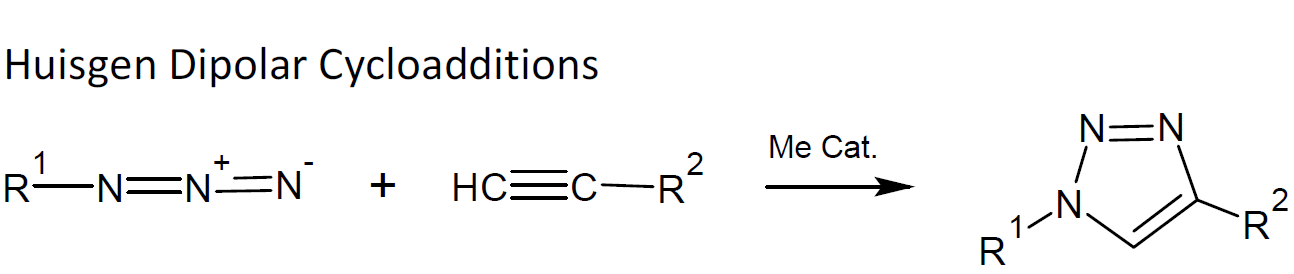 |
 |
 |
 |
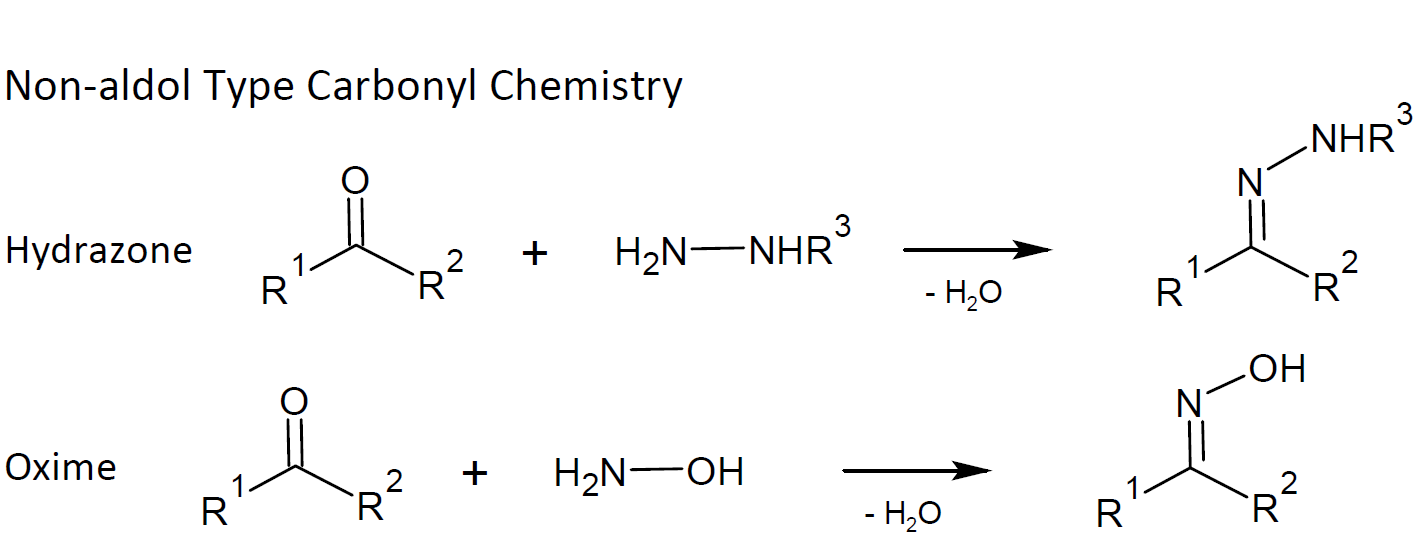 |
The majority of reactions that meet the click requirements are carbon-heteroatom bond forming reactions. Sharpless et al. identified four major classes of reactions that meet their criteria of click chemistry:1,2
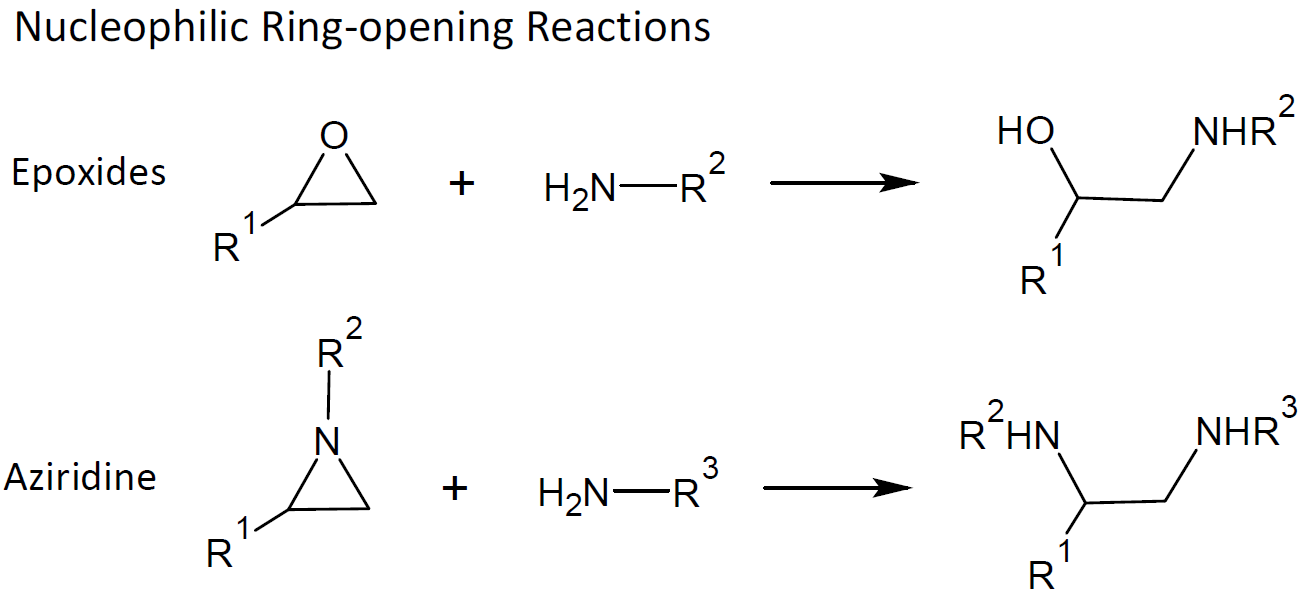
nucleophilic substitution chemistry, mainly ring-opening reactions of strained heterocyclic compounds such as epoxides, aziridines and cyclic sulfates
non-aldol type carbonyl chemistry including the formation of oximes, hydrazones, and aromatic heterocycles
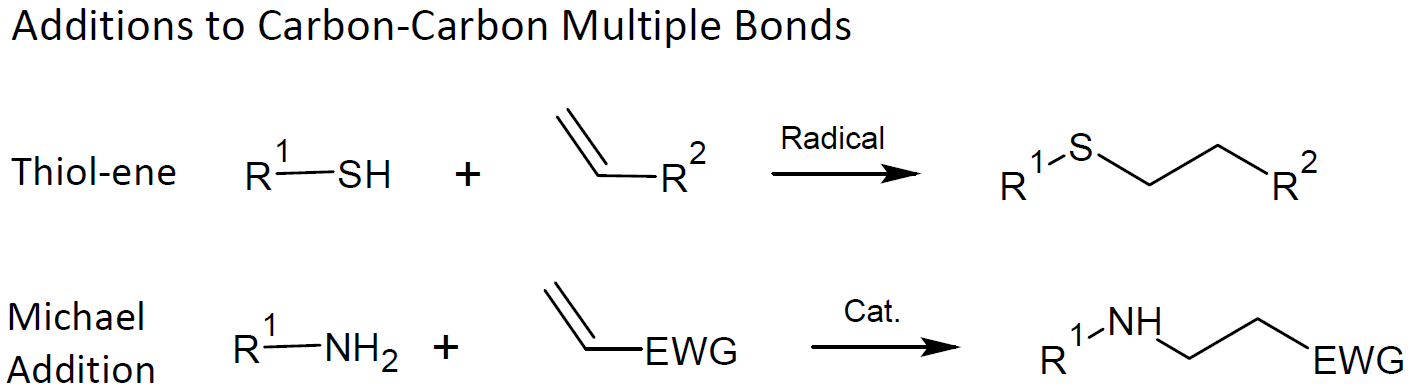
additions to carbon-carbon multiple bonds, especially Michael additions such as amine-ene, thiol-ene and thiol-yne addition but also sulfenyl halide and nitrosyl-halide addition
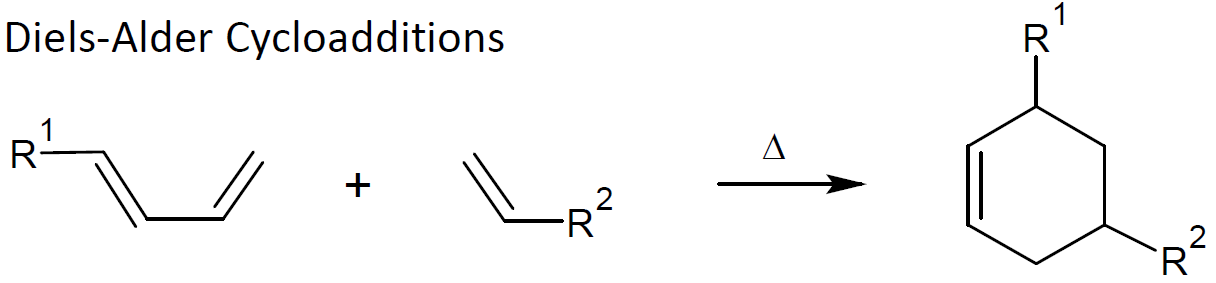
cycloaddition of unsaturated compounds, mainly Diels-Alder reactions and Huisgen 1,3-dipolar cycloaddition
Two other types of reactions that meet most of the click requirements are isocyanate-thiol and isocyanate-amine reactions which produce thioureas and ureas. Both have high reaction rates and very high yield and can be conducted in no or only benign organic solvents. However, isocyanates are water sensitive, that is they react with water to urea (-NH-C(=O)-). Nevertheless, isocyanate-thiol and isocyanate-amine reactions have been considered as part of click chemistry since the presence of a small amount of water does not shut down the reaction.1,6
One of the most powerful click reactions is copper-(I)-catalyzed 1,3-dipolar cycloaddition of azides to terminal acetylenes yielding triazoles (CuAAC).3 This type of reaction is commonly known as the Huisgen dipolar cycloaddition (HDC).5 It is one of the most versatile and reliable click reactions and is remarkably tolerant to many functional groups such as hydroxyl, carboxyl, and amino and thus can be utilized to introduce functional groups into conjugated molecules. This click reaction "is about as good as a reaction can get"1 Not surprising, HDC has been extensively utilized by chemist in the synthesis of novel compounds (drug discovery) as well as for surface functionalization. However, HDC has found very few applications in polymer synthesis due the relative high cost of azides and due to safety concerns (some organic and other covalent azides are toxic and explosive or may react with other compounds to form explosives).
Another very efficient and highly specific click reaction is ring opening of aziridines by nucleophiles such as thioethers. This reaction is mainly used in the synthesis of linear or branched polyethyleneimines (PEI) and in modification of polymers bearing aziridine moieties.14 Ring-opening reactions of epoxides, on the other hand, play a much more important role in polymer chemistry. In fact, epoxy resins are used across almost every industry. The base catalyzed ring-opening polymerization of epoxy groups by nucleophiles such as thiols readily leads to the formation of β-hydroxythioether linkages. This reaction is efficient, regioselective, results in high yield, and is very fast. Furthermore, it generates a reactive hydroxyl group which can also react with oxirane rings; however this reaction is much slower. Thiol-epoxy reactions can also be employed to synthesize functional materials and to crosslink polymers.
A relative new method for the preparation and modification of polymers is nucleophile-initiated Michael addition to carbon-carbon multiple bonds, especially primary amine-ene, thiol-ene and thiol-yne additions.7-13 Thiol-ene and thiol-yne click reactions are mainly utilized to (in situ) modify and functionalize polymers and material surfaces or to create novel polymer architectures (e.g. dendrimers). Both the modular nature and high efficiency has led to an increased interest in thiol-Michael addition chemistry in recent years. Examples include thiol-(meth)acrylate, thiol-acrylonitrile, thiol-vinyl sulfone and thiol-maleimide.12 Michael addition reactions can also be combined with quasi-living radical polymerization techniques (RAFT ) to functionalize polymers by either end or pendant groups allowing the synthesis of well-defined macromolecular structures.7,13
Diels-Alder cycloaddition (DAC) is another popular click reaction which meets most of the click requirements including high efficiency, versatility, and selectivity. The reaction involves the simultaneous formation and destruction of carbon-carbon bonds. Since it has a low energy barrier, it can be carried out even below room temperature. A popular DAC reaction is the addition of furan rings to maleimides. This reaction is fully reversible and can be employed to create self-healing polymer networks.9 Like thiol-ene addition, DAC can be combined with living/controlled polymerization techniques such as RAFT to functionalize polymers and to create complex polymer architectures.
References & Notes
H.C. Kolb, M.G. Finn, K.B. Sharpless, Angew. Chem. Int. Ed., 40, 2005-2021 (2001)
H.C. Kolb and K.B. Sharpless, Drug Discovery Today, Vol. 8, No. 24, 1128-1137 (2003)
The Cu(I)-catalyzed version of Huisgen's cycloaddition of azides to terminal alkynes4 is about 106 faster than the uncatalyzed HDC.1,2
V.V. Rostovtsev, L.G. Green, W. Fokin, K.B. Sharpless, Angew. Chem. Int. Ed. Engl. 41, 2596-2599 (2002)
R. Huisgen, Angew. Chem. Int. Ed., 2, 565 (1963)
Srinivasan Chandrasekaran, Click Reactions in Organic Synthesis, Wiley-VCH, Weinheim, Germany 2016
C.R. Becer, R. Hoogenboom and U.S. Schubert, Angew. Chem. Int. Ed., 48 (2009)
Q. Wang, T.R. Chan, R. Hilgraf, V V. Fokin, K.B. Sharpless, M.G. Finn, J. Am. Chem. Soc. 125, 3192 (2003)
M.M. Diaz a, G. Van Assche, F.H.J. Maurer, B. Van Mele, Polymer, 120, 176-188 (2017)
M.J. Kade D.J. Burke, C.J. Hawker, J. Polym. Sci. Part A: Polym. Chem.: Vol. 48, 743-750 (2010)
B. Yao, J. Li, J. Wang, H. Wu, J.Z. Sun, A. Qin, B.Z. Tang, Macromol. 47, 4, 1325-1333 (2014)
D.P. Nair, M. Podgorski, S. Chatani, T. Gong, W. Xi, C.R. Fenoli, C.N. Bowman, Chem. Mater. 26, 1, 724-744 (2014)
Andrew B. Lowe, Polymer 55 (22), pp. 5517-5549 (2014)
H.J. Jang , J.T. Lee , H.J. Yoon, Polymer Chemistry, 6 (18), 3387-9331 (2015)