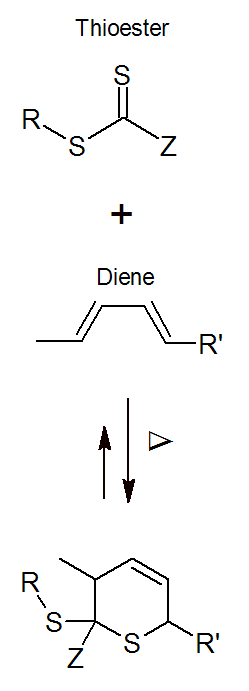
Diels-Alder Cycloaddition
The Diels-Alder (DA) reaction is one of the most common and important reactions used in organic chemistry to synthesize cyclic compounds. It was discovered by Otto Diels and Kurt Alder who received the Nobel Prize for their discovery in 1950. The Diels-Alder reaction is a [4 + 2] cycloaddition of an electron rich conjugated diene to an electron poor dienephile:
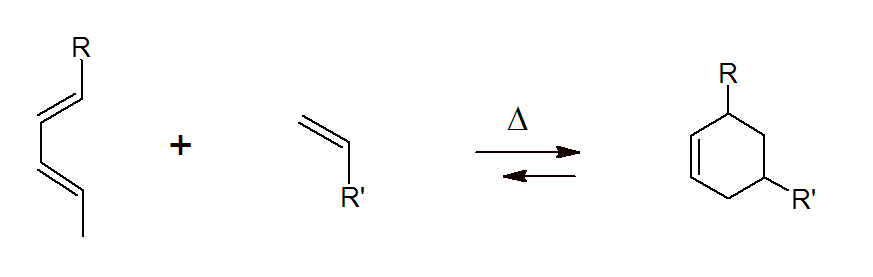
The method has also been applied to other pi-systems, such as carbonyl, imines, and thiocarbonyl, to produce heterocycles, known as hetero-Diels-Alder reaction. Both hetero and homo Diels-Alder reactions are highly regio- and stereoselective1,2 and thus, are very useful for the synthesis of complex molecules which explains the popularity of DA addition reactions among chemists. Furthermore, many DA reactions are reversible at elevated temperatures which is known as retro-Diels-Alder reaction.
In the case of unsymmetrical dienophiles, there are two possible transition states, called endo and exo. In the endo orientation, the cyclic molecules approach each other with maximum overlap (C-shape) and in the exo orientation, with minimum overlap (Z-shape):

The endo transition state is usually preferred in DA reactions that involve electron-withdrawing substituents despite the greater steric hindrance. This preference is known as the Alder rule. The endo preference is often explained by secondary orbital interaction between the π system of the dienophile and that of the diene.
Diels-Alder cycloaddition can be employed to prepare polymers of various architectures. The monomers of this type of
step-growth polymerization are either molecules that contain both diene and dienophile functional groups in a single molecule (A-B type monomers) or they consist
(of stoichmetric amounts) of bisdienes and bisdienophiles (A-A and B-B co-monomers). Some well known examples of A-B type DA reactions in polymer science include the [4+2] cyclo-addition of furan to maleimides and of dithioester to (substituted) dienes.3
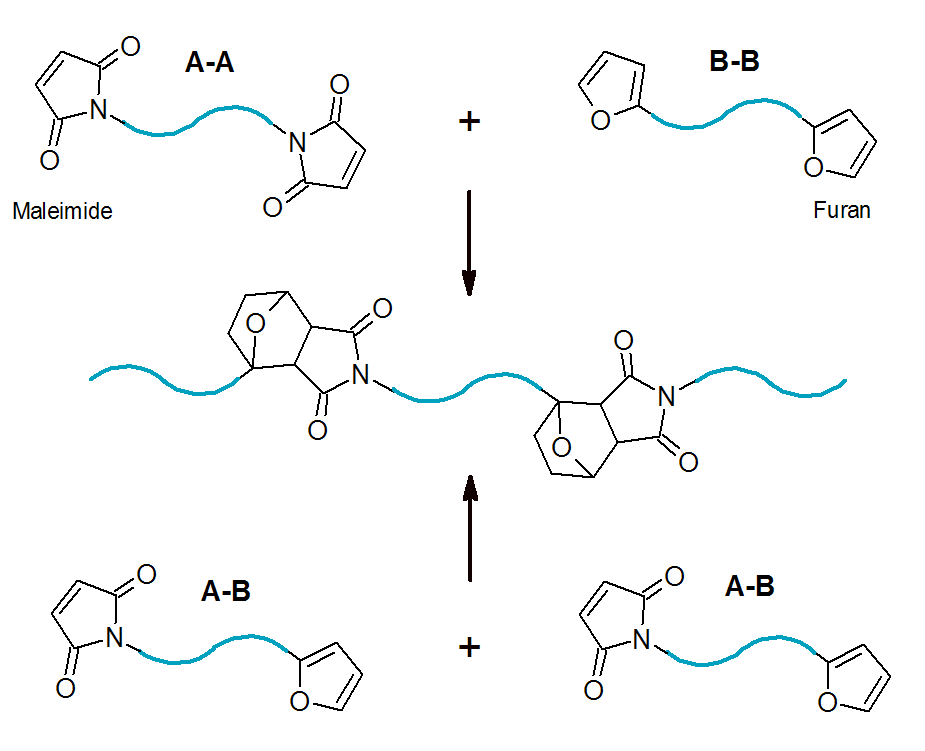
The combination of Diels-Alder reactions with living/controlled polymerization methods has been employed to synthesize complex polymer architectures including dendrimers, stars, ladder, and graft polymers. Due to its simplicity, versatility and efficiency, DA is one of the preferred routes for synthesizing complex polymers.
References & Notes
Regioselectivity Is the preference of certain reaction sites over others. Markovnikov addition reactions are typical examples of regioselectivity. In this addition reaction, the hydrogen atom of a protic acid HX bonds to the carbon atom in an alkene/alkylene that has the greatest number of hydrogen atoms (least substituted carbon)..
In stereospecific reactions, the reactants determine which stereoisomer is (preferentially) formed. Diels-Alder reactions are known to retain the stereochemical arrangement of the substituents, meaning cis/trans (Z/E) dienophiles maintain their stereochemistry in the adduct.
-
Mehmet A. Tasdelen, Polym. Chem., 2, 2133-2145 (2011)
-
I. C.Y. Hou, Y. Hu, A. Narita and K. Muellen, Poly. J., 50, 3-20 (2018)