Ring-Opening Polymerization
by Monomer Activation
Classical ionic polymerization proceeds by a chain growth mechanism which involves the addition of neutral monomers to a positively or negatively charged growing chain end. This mechanism is known as an active chain-end mechanism (ACEM). In the case of ring-opening polymerization (ROP), an activated monomer mechanism (AMM) is also possible, in which monomers are the active species. Both nucleophilic and electrophilic pathways exist. Examples of the two activated monomer mechanisms are cationic ring-opening polymerization of cyclic ethers (see scheme a) below) and anionic ring-opening polymerization of lactams (see scheme b). In both cases, the activated monomer is created by an acid-base reaction which, in a subsequent step, attacks either a monomer (initiation) or a neutral chain end:
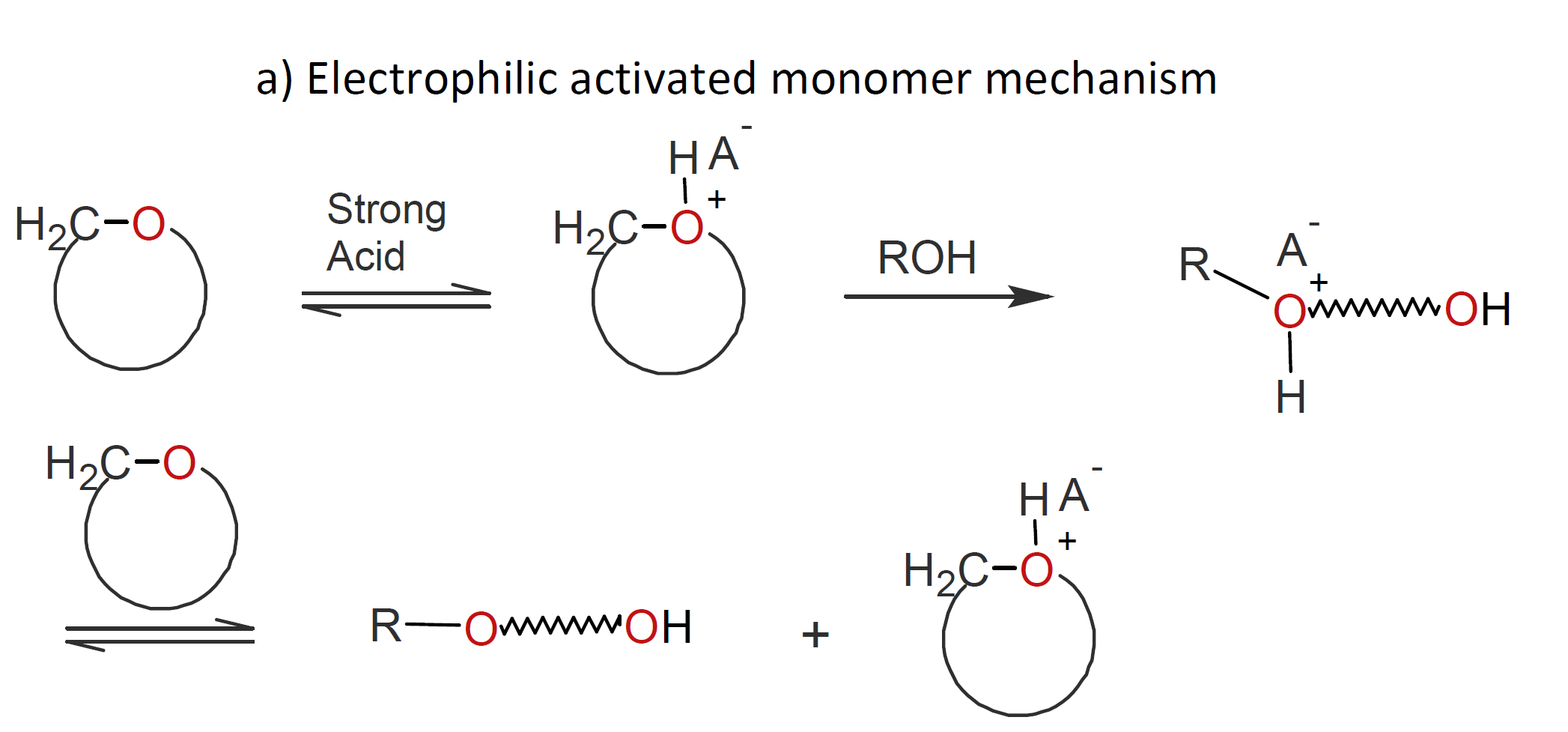
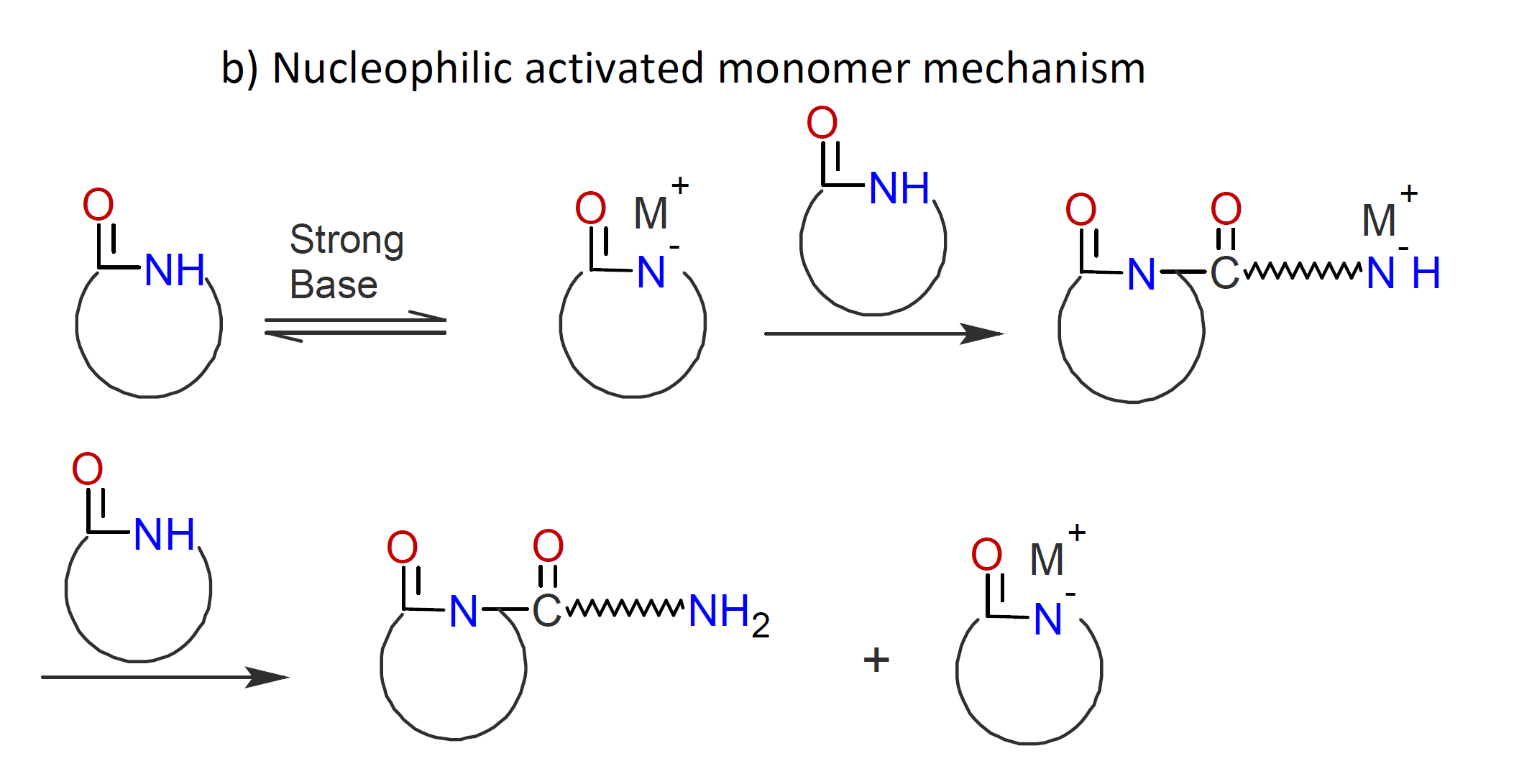
The activated monomer mechanism (AMM) of AROP has been successfully applied to the synthesis of
Nylon 6 and Nylon 12 and
AMM of CROP to the synthesis of polyglycidol and polyethylene oxide.1-6
The activated monomer mechanism sometimes competes with the active chain-end mechanism. Which of the two mechanisms prevails, will depend on the reaction conditions, as well as on the type and concentration of monomer, catalyst and solvent. For example, Kubisa et al. found that in the presence of alcohol, the mechanism of polymerization of cyclic ethers shifts from an active chain-end mechanism to an activated monomer mechanism:1,2
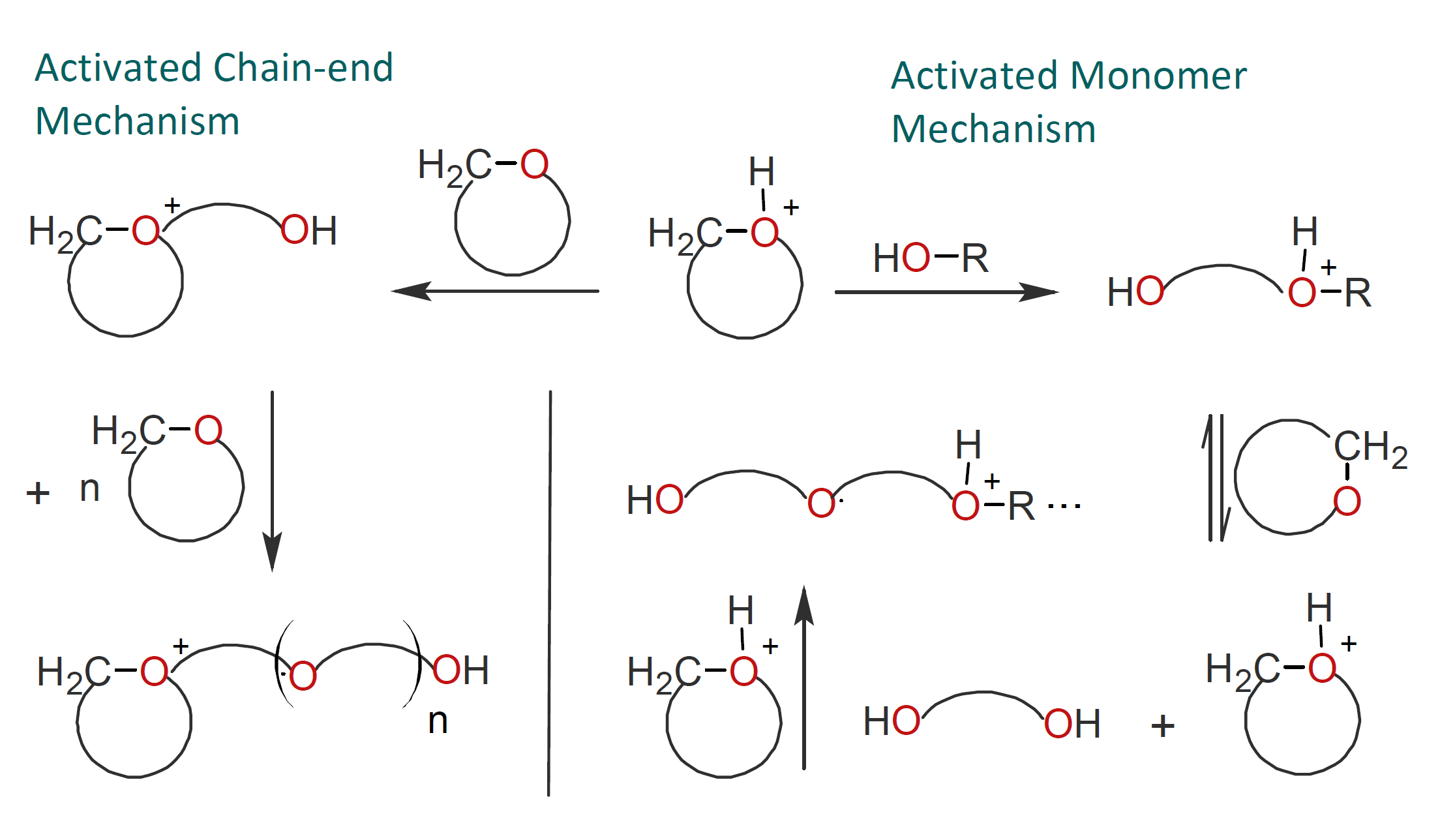
As has been pointed out by Kubisa and others,1,7 some AM-ROPs can exhibit some features of living polymerization. In CROP of cyclic ethers, for example, this is the case when protic solvents such as alcohols and water act as strong transfer agents rather than terminating agents, which leads to fast exchange of protons between the active and dormant chain ends, and when dormant chain ends prevail. Under such conditions, a narrow polydispersity can be achieved.8
The extent to which the AM mechanism contributes to chain growth will depend on the rate of reaction between hydroxyl groups and protonated monomers and that between protonated and neutral monomers:1
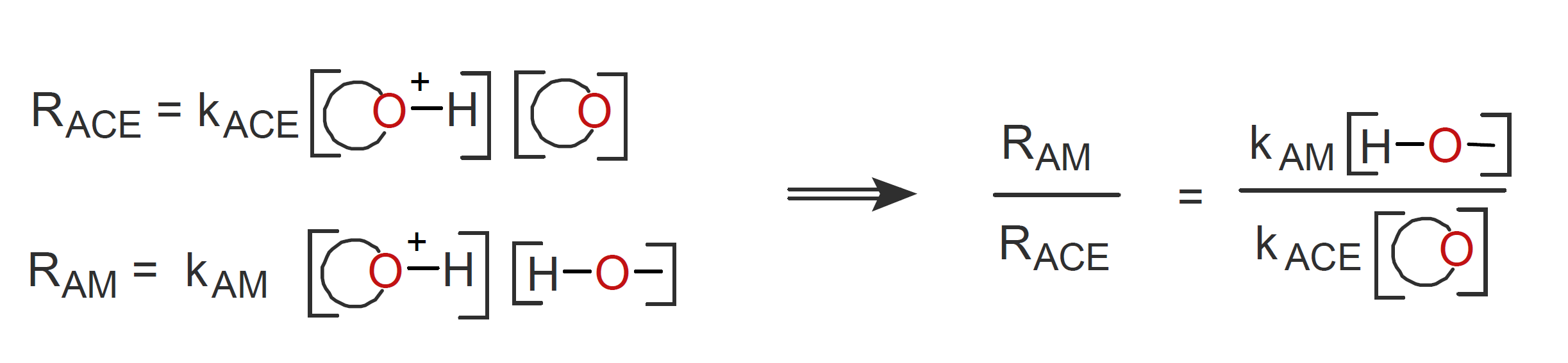
Thus, an increasing hydroxyl group to monomer ratio will favor the AM mechanism, and thus the living character of CROP. The degree of polymerization, on the other hand, depends on the ratio of monomer polymerized to hydroxyl groups initially added because alcohol acts as an initiator and protic acid only as a catalyst.1,14 Thus, to obtain polymers with high molecular weight (and low polydispersity) by an AM mechanism, monomers have to be added steadily and slowly to maintain a low monomer to hydroxyl ratio. This approach also avoids the formation of cyclic polymers due to the absence of intramolecular back-biting, which can be a major problem of active chain-end polymerization because it lowers the average polymer molecular weight and also decreases the rate of polymerization. As has been shown by Kubisa et al., the presence of alcohols and suitable catalysts almost completely eliminates cyclization in cationic polymerization of cyclic ethers.9-11
In the case of caprolactam, the monomer is activated by a strong nucleophile which abstracts a proton from the amine group in the ring. The activated lactam anion then attacks another lactam molecule to induce ring-opening. In a third step, the formed amino-acyllactam anion abstracts a H-N proton from another lactam molecule which produces another activated lactam anion, which, in turn, reacts with an N-acyllactam and so on:2,3
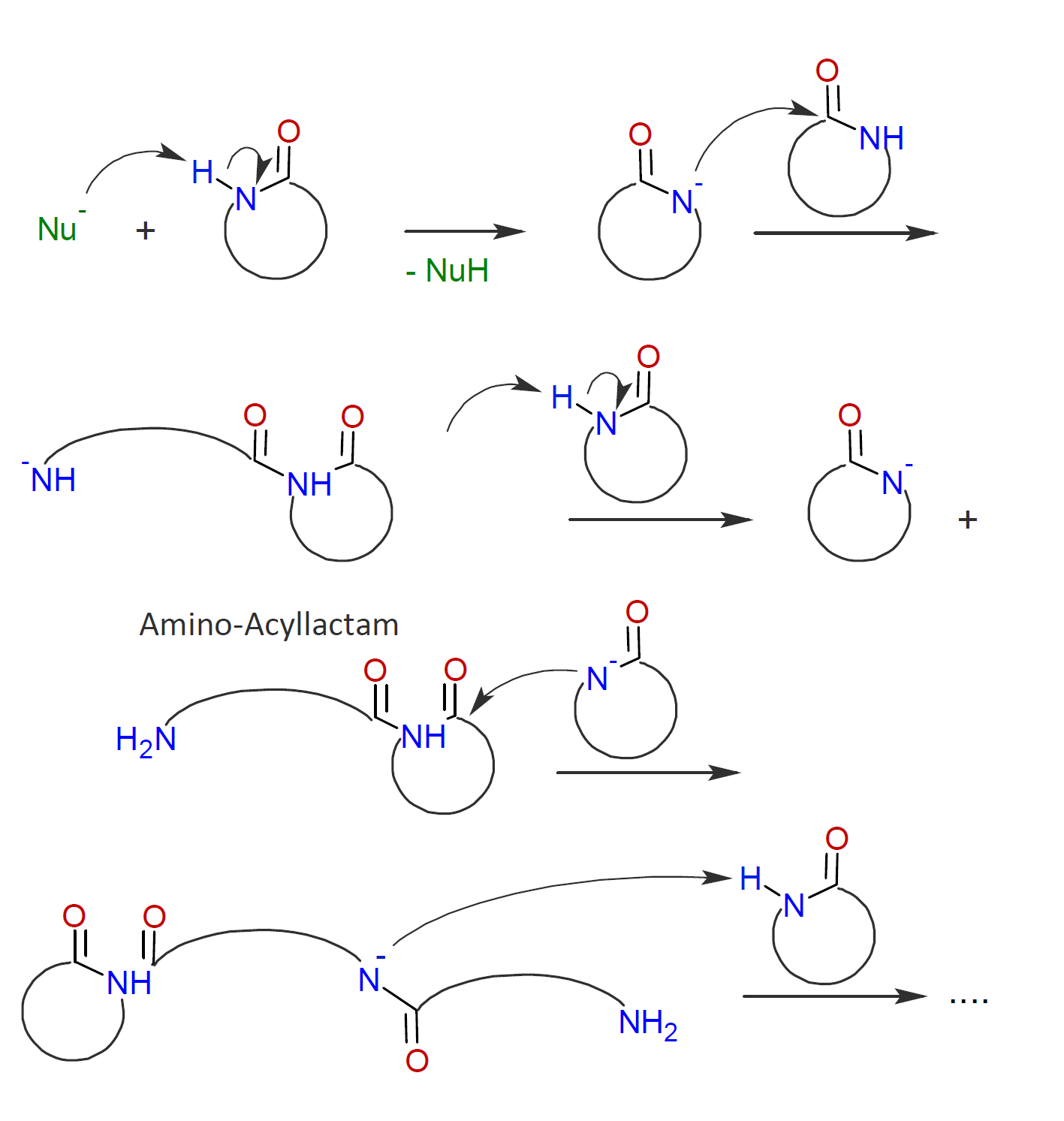
AM-ROP of lactams requires strong base initiators and is typically limited to the more reactive lactams such as caprolactam whereas the less reactive lactams polymerize only sluggishly.12,13 The rate of reaction can be greatly increased with acylating agents such as acid chlorides, anhydrides, isocyanates or monocarbodiimides::12
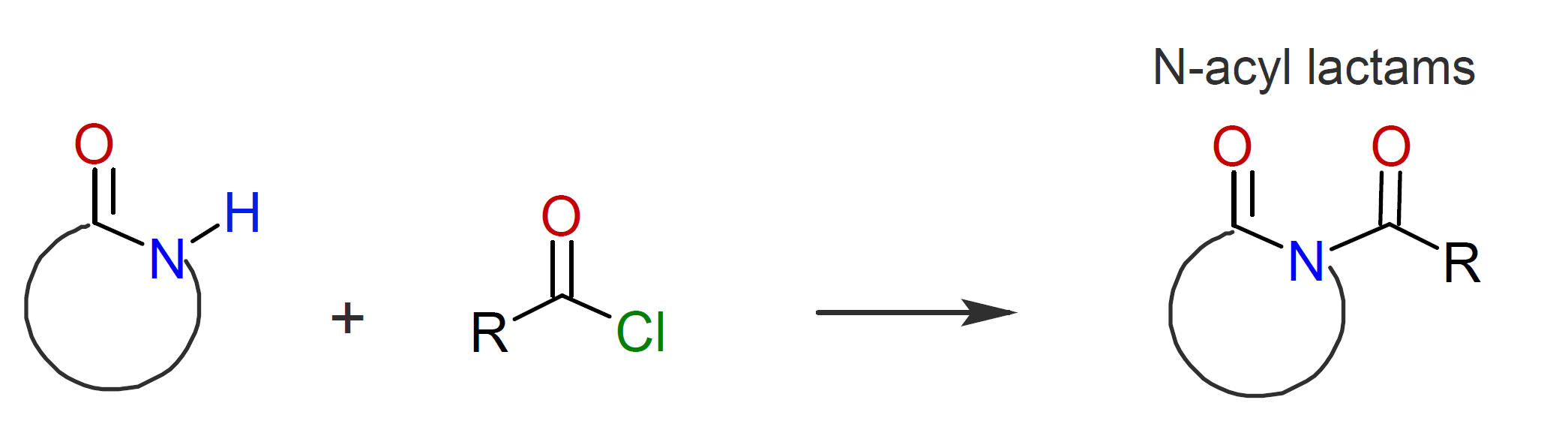
This method often proceeds without a noticeable induction period. In a second step, the N-acyllactam reacts with an activated monomer followed by fast proton exchange with another monomer:
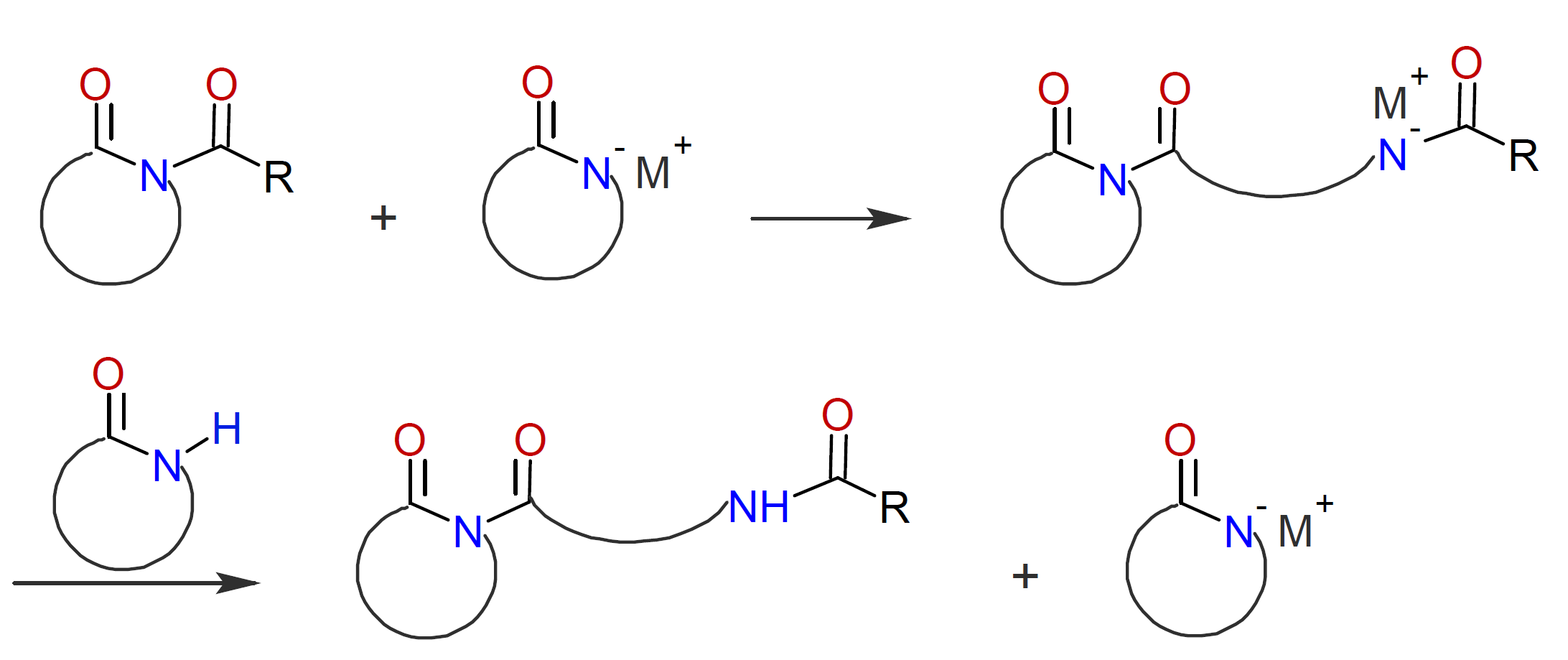
The propagation step proceeds in the same way as strong base initiated AROP except that the propagating chain end has an acylated end group instead of an amine end group:
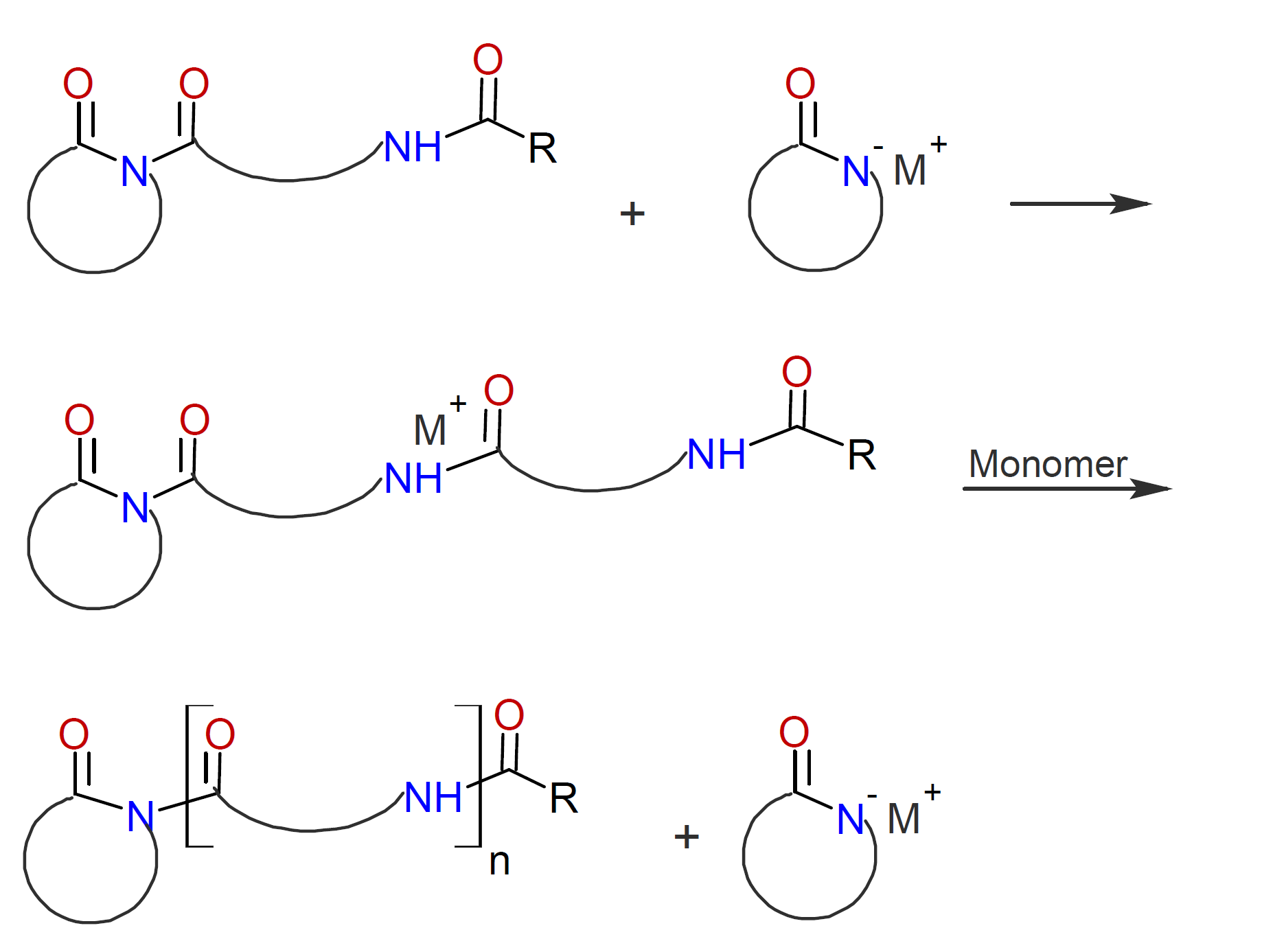
The reaction rate depends on both the type and amount of base and acyl lactam. The rate increases with monomer concentration but decreases with the N-acyl lactam concentration.
Notes & References:
1P. Kubisa, J. Polym. Sci.: Part A: Polym. Chem., Vol. 41, 457–468 (2003)
2P. Kubisa, S. Penczek, Prog. Polym. Sci., 24 (10), 1409–1437 (1999)
3R. Brzezinska, P. Szymanski, P. Kubisa, S. Penczek, Makromol. Chem. Rapid Commun. 7, 1-4 (1986)
4O. Nuyken 1 and S.D. Pask, Polymers 5, 361-403 (2013)
5C. Bakkali-Hassani, Polymerization by monomer activation: application to the synthesis of polyaziridines and polyamides. Polymers. Universitéde Bordeaux (2018)
6A. Dworak, S. Slomkowski, T. Basinska, M. Goesecka, W. Walach, B. Trzebicka, Polimery, 58 (9), 641-649 (2013)
7K. Matyjaszewski, S. Penczek, J. Polym. Sci. Part A: Polym. Chem., 12, 1905 (1974)
8In controlled polymerization with a reversible activation-deactivation mechanism of growing chain ends, all growth centers have an equal likelihood to be active or dormant. Thus, MW and polydispersity can be controlled.
9T. Biedron, P. Kubisa, S. Penczek, J. Polym. Sci., Part A: Polym. Chem., 29, 619 ( 1991)
10T. Biedron, K. Brzezinska, P. Kubisa, S. Penczek, Polym. Int., 36, 73 (1995)
11S. Penczek, J. Polym. Sci.: Part A: Polym. Chem., Vol. 38, 1919–1933 (2000)
12Wei-Fang Su, Ring-Opening Polymerization. In: Principles of Polymer Design and Synthesis. Lecture Notes in Chemistry, Vol 82. Springer, Berlin 2013
13Philippe Dubois,Olivier Coulembier,Jean-Marie Raquez, Handbook of Ring-Opening Polymerization, 2009
14Hydroxyl groups are usually detrimental to cationic polymerization because water and alcohols and other strong nucleophiles act as terminating agents.
26 October, 2020, Revised 15 November 2020