Initiation By Peresters
A larger number of substances are capable of generating free radicals and have been found to be effective initiators for the polymerization of vinyl monomers. An important class of initiators1 are peresters, which have the general structure R1C(O)OOCR2, wherein R1 usually represents an aryl group (Ar) and R2 an alkyl group.
Peresters are thermally unstable, decomposing at relative low temperatures, thereby generating free radicals. The thermal decomposition involves the release of CO2 and free radicals as shown below:
ArCH2CO−O−O−CMe3 → ArCH2· + CO2 + ·OCMe3
The reaction is driven by the formation of the very stable CO2 molecule and by the resonance stabilization of the benzyl radical formed. As expected, the greater the resonance stabilization of the radical the lower the temperature of decomposition of the perester.
Due to their instability, peresters are capable to initiate polymerization thermally at room temperature or even below. Although the decomposition results in a highly stabilized aryl radical, it might not be an efficient initiator. However, a t-butoxy radical is also formed which readily initiates polymerization. It is also possible that this radical undergoes disproportionation which yields acetone and a methyl radical which is also an effective initiator,
·OCMe3 → CH3· + Me2C=O
The rate of decomposition depends on the solvent. It increases by electron donating groups and decreases by electron withdrawing groups, because an increase in electron density on the oxygen atoms increases repulsion between them and thus enhances the rate of spontaneous cleavage of the peroxide bond.
The reactivity of peresters depends on the solvent and monomer. In the absence of accelerators, the rate of in initiator decomposition is of first order, and is directly proportional to the initiator concentration:
RI = d[I·] / dt = 2 f kd [I]
where f is the efficiency of initiation defined as
f= rate of initiation of polymerization / 2 x rate of decomposition of initiator
t-Butyl Perbenzoate
One of the most common peresters is t-butyl perbenzoate. It is structurally intermediate between dibenzoyl peroxide (BPO) and di-tertiary-butyl peroxide (DTBD). Not surprising, its thermal decomposition kinetics is inbetween those of the two parent initiators. It readily undergoes fission, forming a benzyl and a t-butoxy radical while releasing CO2:
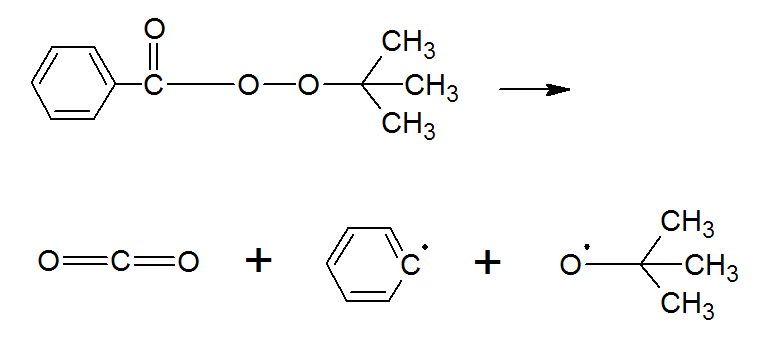
Following its generation, the initiating free radicals react with a monomer thereby creating growing polymer chains.
Another important perbenzoate is tert-amyl peroxybenzoate. Some important aliphatic peresters include tert-butyl and tert-amyl peroxyacetate, and tert-amyl and tert-butyl peroxypivalate.
Notes
The initiators are sometimes erroneously called catalysts. Initiators are consumed in the reaction while catalysts are regenerated after the completion of the reaction.