Initiation By Azonitrile Compounds
Aliphatic azonitriles and related compounds are widely used as initiators1 for vinyl polymerization. They have been first used on a industrial scale as blowing agents in the preparation of light-weight plastics since nitrogen is released during thermal decomposition. The azo compounds have the general formula
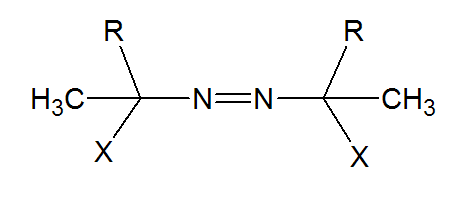
where R is an alkyl group and X a carboxylic acid derivative such as a nitrile or ester group. These compounds contain weak covalent bonds that easily cleave when exposed to heat, producing free radicals and nitrogen gas:
R-N=N-R → 2R· + N2
The by far most important azonitrile is azobisdisobutyronitrile or 2,2'-azodi(isobutyronitrile), abbreviated AIBN. It is widely used as a blowing agent for producing expanded elastomeric polymer products. But it is also one of the most important free radical initiator. When AIBN decomposes, it forms two 2-cyanopropyl radicals with eliminaton of one molecule of nitrogen:
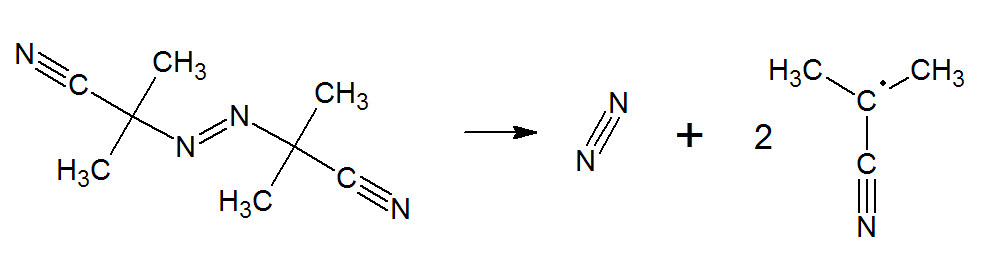
The two 2-cyanopropyl fragments with unpaired electrons are the free radical initiators. Following their generations, they react with a monomer unit thereby creating growing polymer chains.
Other important azonitrile compounds include 1,1'-azodi(hexahydrobenzonitrile), and 2,2'-azodi(2-methylbutyronitrile).
AIBN and some of its derivatives are usually safer to use than benzoyl peroxide (BPO), be-cause the explosion risk is much lower. However, these compounds are still considered explosives.
The decomposition rate of azo compounds, like those of peroxides, are temperature dependent, that is, the rate increases with increasing temperature. The rate also depents on the surrounding medium (solvent).
Numerous lists are available in the literature that give the decomposition temperature for different half-life times or the half-life time for different temperatues (see link on the right sidebar).
Notes
The initiators are sometimes erroneously called catalysts. Initiators are consumed in the reaction while catalysts are regenerated after the completion of the reaction.