Retardation and Inhibition
of Vinyl Polymerization
The rate of polymerization of vinyl monomers can be reduced or almost completely inhibited by the addition of relative small amounts of certain molecules, called retarders and inhibitors. Inhibitors are substances that almost completely suppress the polymerization reaction, that is, the inhibitor has to be completely consumed before the reaction rate assumes its normal value. The induction time, which is the time between addition of initiator and start of reaction (normal rate of reaction), is linear proportional to the amount of inhibitor added. Retarders, on the other hand, only reduce the rate of polymerization, that is, the rate of reaction steadily increases as the retarder is consumed. The equivalent induction time of a retarder is the time that would have been required for all the retarder to be consumed if it had completely suppressed the reaction.
According to the general theory of inhibition, inhibitors and retarders react with initiator radicals to give products that are unable to induce further polymerization; retarders are less reactive than inhibitors and, therefore, do not entirely prevent initiators from reacting with monomers followed by the propagation reaction. Thus, a high concentration of retarder will simulate the behavior of an inhibitor.
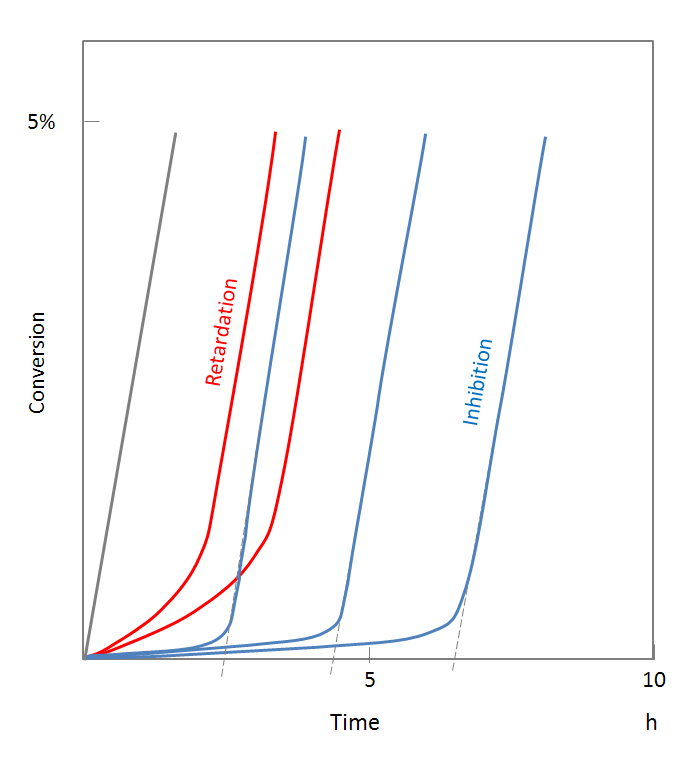
Typical conversion time curves are shown below. The polymerization in the presence of an inhibitor (blue curves) shows a flat plateau with no or very low reaction rates; at a certain point in time (induction time) the reaction rate rapidly increases to a value close to the one found in the absence of inhibitor. The retarder, on the other hand, reduces only the rate of polymerization (red curves). The reaction rate increases steadily as the retarder is consumed and finally assumes its normal rate.
Inhibition Versus Retardation
The unintentional presence of retarders is often the root cause for the irreproducible rates of reaction of incompletely purified monomers, that is, certain impurities can act as retarders or inhibitors. In fact, it has been observed that a wide variety of substances show a moderate retarding effect, whereas only a small number of compounds strongly retard or inhibit polymerization.
Typical inhibitors are quinones and their derivatives and sterically hindered phenols. These compounds strongly inhibit the polymerization of vinyl monomers. Considerable induction times can be achieved with relative small amounts of quinones. The main purpose of these compounds is to improve the shelf-life of reactive monomer systems like curable coatings and adhesives or to improve the heat and UV stability of polymers, i.e. they act as stabilizers. They can also be added at larger concentration to delay cure, i.e. to adjust the open-time and pot-life.
Common retarders are nitro- or nitroso-derivatives of aromatic compounds. These molecules have a strong retarding effect on many vinyl monomers, and in a few cases, act as inhibitors. A prominent example for the later is vinyl acetate. Other strong retarders or inhibitors for many vinyl monomers are oxygen, iodine, and sulfur. Many investigations suggest that sequences of
-[-M-O-O-]- or -[-M-S-S-]-
appear in the polymer which are produced by a reaction of the form
R· + O2 → ROO·
These chain radicals preferentially undergo chain termination, but propagation also occurs because oxygene and sulfur have been found in the polymer as well, however at a relative low concentration. Thus oxygen in the air can inhibit polymerization, particularly at the air-monomer interface. This phenomenon is widely known as air inhibition.