Ozonation reactions and Ozone Cracking in Elastomers
Both ozone and sunlight rapidly attack unprotected polymers which can significantly reduce the service life of a plastic. Particularly polymers with high unsaturation (i.e. rubbers) will suffer from ozone degradation, because the double bonds in unsaturated polymers readily react with ozone. However, ozone also reacts with saturated polymers but at a comparatively slower rate.The reaction of ozone with double bonds causes chain scission. The general mechanism of ozone degradation is shown below.
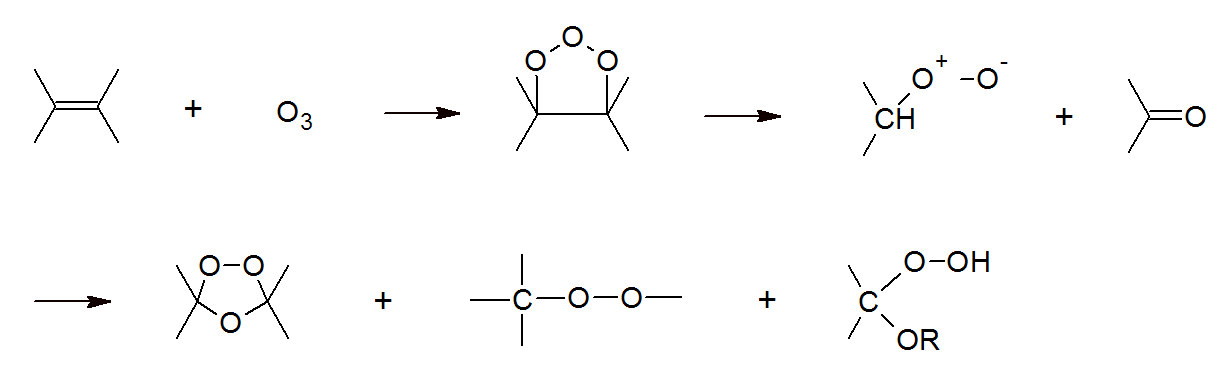
Chain scission and oxidation causes a decrease in cross-link density (elastomer), or molecular weight and molecular weight distribution (thermoplast), and a change in composition (oxidation). The result is a more or less steady decline in the (mechanical) properties.
The aging is greatly accelerated by stress; one usually observes surface cracks in the direction perpendicular to the applied strain when a critical stress value is exceeded. At rather low stress values just above the critical value, long and deep cracks are observed, whereas at high stress values, the ozone cracks become more numerous and are finer in size. The microscopic disintegration of the surface causes dulling and a bluish sheen of the surface of rubber goods. This phenomenon is known as "frosting" because it often resembles actual frost.1 It is greatly accelerated by humidity and heat and is most noticeable on the bright finish of air-cured rubber goods.2 Frosting can be avoided or reduced by certain types of high melting point waxes or by antiozonants such as para phenylenediamines (PPDs) and derivatives thereof (Dialkyl PPD).
In general, the resistance to ozone cracking and frosting will depend on the chemical composition of the polymer. Elastomers are particularly susceptible to ozone attack, particularly those with electron donating side groups (e.g. methyl groups in isoprene), whereas rubbers with electron-withdrawing side groups (e.g. chlorine in neoprene) are noticeably less susceptible to ozone attack due to the deactivating effect of the halogen on the double bond.
The degree of ozone degradation will depend on the composition of the atmosphere and temperature. Usually, the ozone concentration is rather low.3 Nevertheless, even low values of ozone at ambient temperatures can cause significant degradation over time.
1Frosting should not be confused with blooming which is caused by migration to the surface of certain constituents of the rubber composition which can often be removed by heating to the vulcanization temperature
2W.F. Tuley, Ind. Eng. Chem., 31 (6), pp 714-716 (1939)
3The average ozone concentration is about 5 to 10 pphm. Howeever, the actual value can fluctuate and depends on weather conditions, geographic location, and is strongly affected by pollution; in unpolluted areas the ozone concentration is about 1 to 50 pphm, whereas in polluted areas it can reach much higher values of more than 100 pphm.