Nanoparticle-Reinforced Polymers
Nanoparticle-reinforced polymers have gained a lot of attention lately due to the unusual viscoelastic behavior of these systems. In polymer nanoparticle composites, even very weak interactions between the particles and the polymer repeat units noticeably effects the composite properties. In the case of attractive interactions, the polymer molecules will coat the particles. The random polymer coils will stretch out at the polymer-particle interface and change to a tail-train-loop structure. The average size of the loops, trains, and tails depend on the flexibility of the chains and the adsorption energy. The adsorption process is rather fast and in the beginning the polymer composition at the polymer-particle interface will be similar to that of the polymer matrix. However, the shorter chains will be slowly replaced by longer chains which will effect the layer thickness and composite properties. The polymer coated filler particles resemble core-shell particles, consisting of a hard core and a soft shell of more or less immobilized polymers. The thickness of the shell, δ, will depend on the solvent and particle properties. In good solvents, the layer thickness scales with M3.5 and in theta-solvents and in polymer melts with M1/2.
The effect of nanoparticles on polymer rheology is rather complicated and can not be fully explained by a free volume effect alone. As has been shown by Mackay and others the viscosity strongly depends on the average distance (H) between particles and their volume fraction φP 2-4:
H / 2r = [φP,m / φP]1/3 - 1
where φP,m is the maximum packing density (≈ 0.638) and 2r is the particle diameter. In the case of polymer coated particles the particle volume fraction has to be replaced by the effective particle volume fraction:
φP,eff = φP,m [1 + δ/r]1/3 ≈ φP,m [1 + 3δ/r]
Due to the very high surface to volume ratio of nanoparticles, a large amount of polymer can be adsorbed and immobilized by the particles. Assuming a volume fraction φP = 0.1 and assuming an excluded volume layer of δ = 0.1 nm exist around each particles with a radius r = 3 nm, the fractional volume within the polymer matrix is increased by ΔφP = 3 φP,m δ/r = 0.01 which is a 10 percent increase in particle volume whereas the volume increase in typical colloidal suspensions with particles sizes of several hundred nanometers is very small.
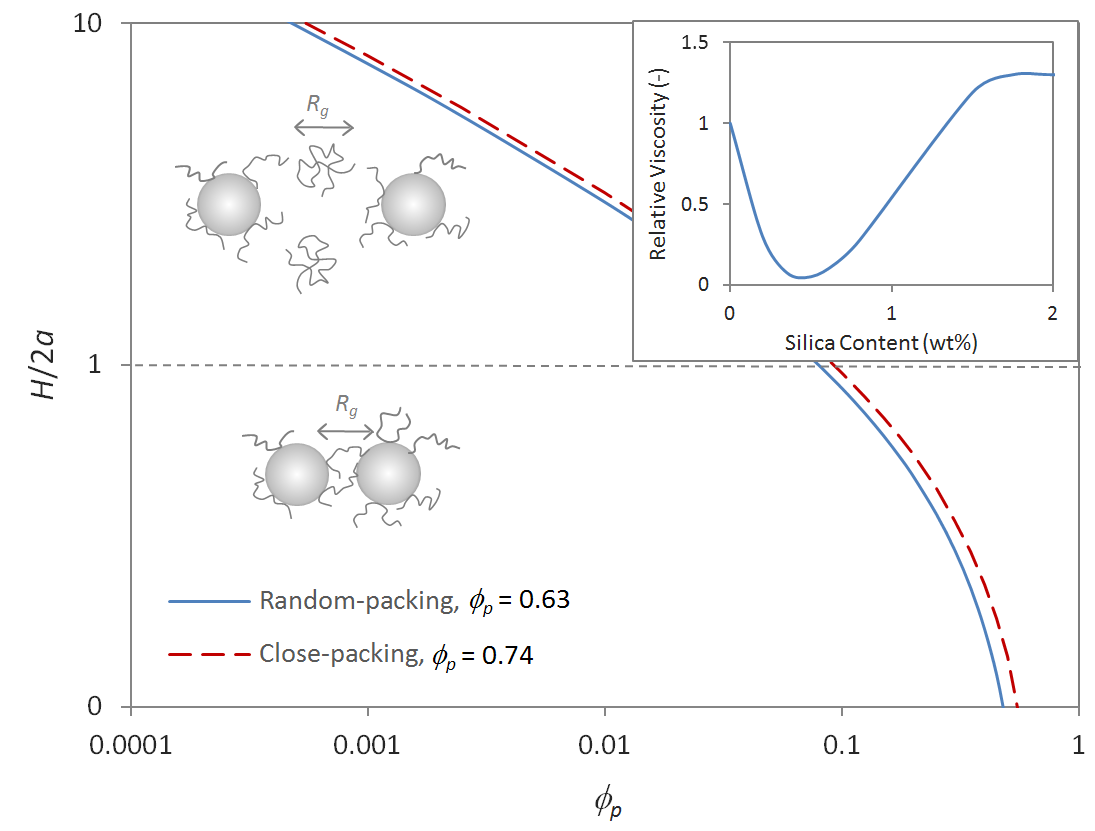
Both the interparticle distance H and the size of the polymer coils Rg (radius of gyration) affect the viscoelastic behavior of nanocomposites. Assuming random packing of the fully dispersed particles, three different regimes can be defined:
H < 2Rg: This the case at low filler levels. In this regime, polymer bridging can be neglected and the suspension shows normal behavior.
H = 2Rg: This is the critical distance where deviations from normal suspension behavior can be expected. In this regime a polymer will be able to touch and bridge two particles without undergoing large deformations (change of entropy).
H > 2Rg: At high filler loadings the average gap between filler particles is smaller than the polymer coils. This situation leads to extensive bridging and polymer confinement.
A noticeable drop in viscosity in weakly interacting polymer-nanoparticle system is observed when the confined layer thickness H decreases below the average size of the molecules Rg. This unusual behavior can be explained by a reduced entanglement density; the polymers in the confined space have to stretch in the direction parallel to the interface to fit between the dispersed particles. This leads to a reduced entanglement density in the confined space because stretched molecules are less likely to entangle and therefore a lower viscosity can be expected. This phenomenon is only observed in systems where the particle size is comparable to or somewhat smaller than the size of the polymer coils. If the particle distance decreases below a certain point, polymer molecules will be able to bridge particles, that is they will be attached to two particles simultaneously. This gel-effect together with strong frictional forces within the polymer dispersion lead to a noticeable increase in viscosity. The behavior at small particle-particle distances depends on the magnitude of the adsorption energy. In the case of strong interfacial forces, the polymer molecules lie flat on the particle surface so that particle bridging is shifted to higher particle concentrations. The behavior at larger distance is less predictable. Both an increase in viscosity (classical behavior) and a decrease in viscosity has been reported. It was found that the visocelastic properties depend on the particle size and the molecular weight of the polymer as well as on the interaction between the particles and the polymer molecules. For example, in nanocomposite with strong particle-polymer interaction, many trapped entanglements form near the particle surfaces which cause a local drag during deformation and viscose flow and a slower disentanglement under deformation. This, in turn, leads to a higher viscosity and a higher critical strain. In the case of weak interaction between particles and polymer such as polypropylene and silica, a continuous decrease in viscosity with increasing particle concentration (at less than 1 wt%) has been reported (see figure above).4 The reduction in viscosity at low particle loadings is the result of reduced entanglement whereas at higher concentrations the increase in viscosity is caused mainly by polymer bridging and not so much by hydrodynamic forces.
Many other properties are effected by nanoparticles. For example, nanoparticles change the phase formation during crystallization. As one might expect, the degree of crystallinity increases whereas the average spherulite size decreases.5 The addition of nanoparticles also accelerates crystallization due to the nucleation effect of nanoparticles. However, this is not always the case; depending on the concentration and filler type, the nanoparticles can either increase or decrease the degree of crystallinity. The decrease is the result of selective adsorption and polymer bridging of particles. Both leads to lower chain mobility and thus slower crystal growth. In general, the highest crystallization rate is observed at the lowest viscosity. Thus, a smaller amount of nanoparticles might be more beneficial to crystal growth than a large amount.
Nanoparticles also affect the viscoelastic behavior of solid polymers. The addition of small amounts (often less than 5%) increases the modulus and yield stress due to reinforcement and interaction between the nanoparticles and polymer molecules. In some cases, the viscoelastic and thermo-mechanical properties will change dramatically. These changes are often the result of increased crystallinity and/or smaller crystals. Nanoparticles are also known to increase the impact strength.4,6. The increase in fracture toughness is the result of stress-induced cavitation (debonding of polymer matrix from the polymer particles) followed by plastic deformation around the voids as well as shear banding. However, the improvements are not as significant as in fiber-reinforced systems.
References
- C.G. Robertson, R. Bogoslovov, C.M. Roland, Rubber Chem. Technol., Vol 82, p. 202-213 (2009)
- M.E. Mackay, T.T. Dao, A. Tuteja, D.L. Ho, B.v. Horn, H.C. Kim and C.J. Hawker, Nature Mater., 2, 762-766 (2003)
- A Tuteja, M.E. Mackay, C.J. Hawker and B van Horn, Macromolecules, 38, 8000-8011 (2005)
- S. Jain, G.P. Goossens, G.W. Peters, M. Duin amd P.J. Lemstra Soft Matter, 4, 1848-1854 (2008)
- D. Eiras, L.A. Pessan, Materials Research, Vol 12, No 4, p. 523-527 (2009)
- S. Sprenger, J. Appl. Polym. Sci., 130, 1421-1428 (2013)
- Ana María Diez-Pascual, Polymers, 11, 625 (2019)