Diffusion of Polymer Chains
A small particle diffuses in solution and melt due to fluctuations of the number of nearby molecules randomly colliding with the particle from different directions. Polymer molecules are typically much larger than solvent molecules (or monomers) but still small enough that random collisons with other molecules noticeably move the polymer. The net transport of molecules due to diffusion is typically caused by a concentration or pressure gradient. According to Fick's first law of diffusiion1, the diffusive flux ji (i.e. the amount of substance i diffusing through a unit area per unit time) due to a concentration gradient is proportional to the concentration gradient:
ji = -Di ∇ci
If the concentration varies only in one direction, Ficks first law reduces to
ji,x = -Di δci /δx
where Di is called the diffusion coefficient of substance i and ci is its concentration at x. The rate of change of concentration is given by Fick's second law of diffusion:
δci /δt = -δji,x /δx = Di δ2ci /δx2
And if the concentration depends on all three coordinates,
δci /δt = Di ∇2ci
This is the so-called diffusion equation.
The random motion of a
particle (i.e. its trajectory) resembles a random conformation of a Gaussian polymer chain. In other words, a random motion of a particle describes an ideal polymer chain.
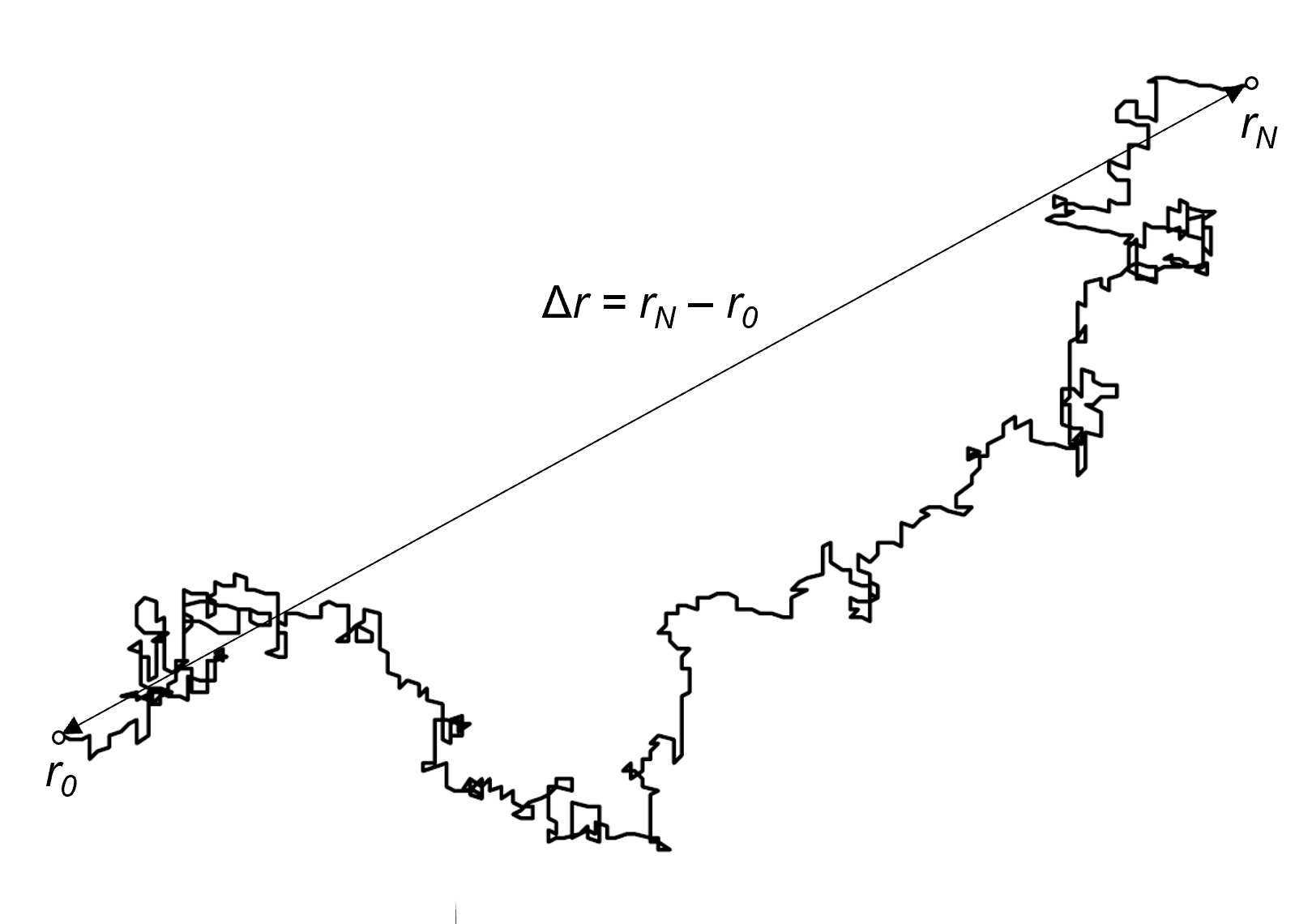
Then the mean square displacement of a N step walk in time t = N t1 is equivalent to the mean-square end-to-end distance ⟨R2⟩ of an ideal polymer chain:
⟨(rN - r0)2⟩ ≅ 〈[r(t) - r(0)2]⟩
The average displacement of a particles in time t is (6Dt)½ whereas the average root-mean-square end-to-end distance of an ideal polymer chain is lN½. Then the Diffusion coefficient is given by
D = ⟨(rN - r0)2⟩ / 6t = Nl2 / (6N t1) = l2 / 6t1
A Gaussian chain has the probability distribution
P(rN, r0, N) = (2 πNl2/ 3)-3/2 exp[-3(rN - r0)2 / (2Nl2)]
Which now can be written as2
P[r(t), r(0), t] = (4 πDt)-3/2 exp{-[r(t) - r(0)]2 / (4Dt)}
and in one dimension:
P[x(t), x(0), t] = (4 πDt)-1/2 exp[-([x(t) - x(0)]2 / (4Dt)]
The figure below shows the probability distribution of a diffusing particle for a one dimensional random walk. The curves are given as a function of [x(t) - x(0)]/l for = 0.1, 0.25, 1, and 10, where l is the unit length. At t = 0, the particle is at x(0). Then P[x(t1), x(0), t1] is the probability that the particle will reach the point x(t1) at time t1.
Probability distribution of a Gaussian Particle

The dynamic of an ideal (Gaussian) polymer chain was first investigated by Rouse in 1953.3 He showed that the motion of a polymer chain in its melt state and in solution can be described with a simple bead-spring model. In this model, the chain is composed of a series of beads and springs. Each spring has a length of l and a spring constant of 3kT/l2. The calculations are rather involved but the results are remarkably simple. According to the Rouse model, the mean square displacement of the center of mass Rg in time t is directly proportional to the time:
〈[Rg(t) - Rg(0)]2⟩ = (6kT / Nζ) · t
where ζ is the friction coefficient of a bead. Using the Einstein relation D = kT / ζ , the diffusion coefficient of the center of mass can be written as
Dcoil = kT / Nζ
Thus the diffusion coeffient decreases inversely with the chain length N (number of beads).
The time correlation of the end-to-end distance of a polymer coil decays like2
〈R(t) R(0)〉 = (8Nl2/ π2) ∑p=1,3,5... p-2 exp(-tp2/τ1) ≈ (8/π2) 〈R2〉 exp(-t/τ1)
where τ1 is the relaxation time of mode p = 1, which is often called the maximum relaxation time of the polymer coil:2,4
τ1 = N2l2ζ / (3π2kT) ≈ Nl2 / Dcoil ≈ ⟨R2〉 / Dcoil
The relaxation time τ1 is typically very short. Thus a polymer chain loses its memory very quickly (exponentially) with a characteristic time τ1. Furthermore, a polymer diffuses a distance of the order of its coil size during the Rouse time τ1:
⟨R2〉1/2 = 〈[r(t) - r(0)2]〉1/2 ≈ (Dcoil τ1)1/2
Rubinstein and Colby3 suggested that the relationship τ1 ≈ ⟨R2〉 / Dcoil is universal, i.e. it should also apply to real chains of size R = l Nν. Then the relaxation time τ follows the scaling relationship
τ1 ≈ ⟨R2〉 / Dcoil = l2N2νN ζ / kT = N2ν+1τ0
where τ0 = ζ l2/ kT is the relaxation time of an individual bead. For time scales shorter than τ0 the polymer behaves like an elastic body. In other words, this time is too short for the polymer to disentangle and to dissipate mechanical energy as heat. If, on the other hand, τ0 < t < τ1, the polymer shows viscoelastic behavior and for very large time scales, t >> τ1, the polymer behaves like an ordinary liquid.
It has been demonstrated experimentally that τ1 of a polymer in a θ-solvent is proportional to N3/2 and not N2. This discrepancy is due to hydrodynamic interactions7 which are not accounted for within Rouse's model. Another model was developed by Zimm5 which correctly predicts τ1≅ N3/2. Zimm assumed that the moving polymer coil drags some of the surrounding solvent molecules with it which increases the friction coefficient ζ. In the case of a non-draining polymer coil, a polymer behaves like a solid particle and Stokes law applies, thus6
ζp ≈ ηs R ≈ ηs lNν
where ηs is the viscosity of the surrounding solvent. Using the Einstein relation D = kT / ζ , the diffusion coefficient of the center of mass can be written as
Dcoil ≈ kT / ηs lNν
Zimm's exact calculation for an ideal chain reads5
Dcoil = 0.192 kT / ηs lNν
Zimm's relaxation time τ is of order
τ1 ≈ ⟨R2〉 / Dcoil ≈ ηsR3/kT ≈ ηsl3N3ν/kT ≈ N3ντ0
which is the time a particle diffuses a distance of the order of its size. The Zimm model is in good agreement with the experimentally observed behavior of a polymer in a dilute solution:
Dcoil ∝ Mν; τ1 ∝ M3ν
References & Notes
Adolf Fick, Annalen der Physik, 170, 59-86 (1855)
Masao Doi, Introduction to Polymer Physics, New York 1997
P. E. Rouse, J. Chem. Phys. 21, 7, 1272-1280 (1953)
Michael Rubinstein and Raph Colby, Polymer Physics, 1st Ed., New York 2003
B. H. Zimm, Journal of Chemical Physics, 24, 269-278 (1956)
Stokes law for a spherical particels of radius R reads ζ ≈ 6π ηR. However, polymers are not spherical. Thus, the numerical prefactor will be be different.