Adhesion Contact Mechanics
Hertz Theory
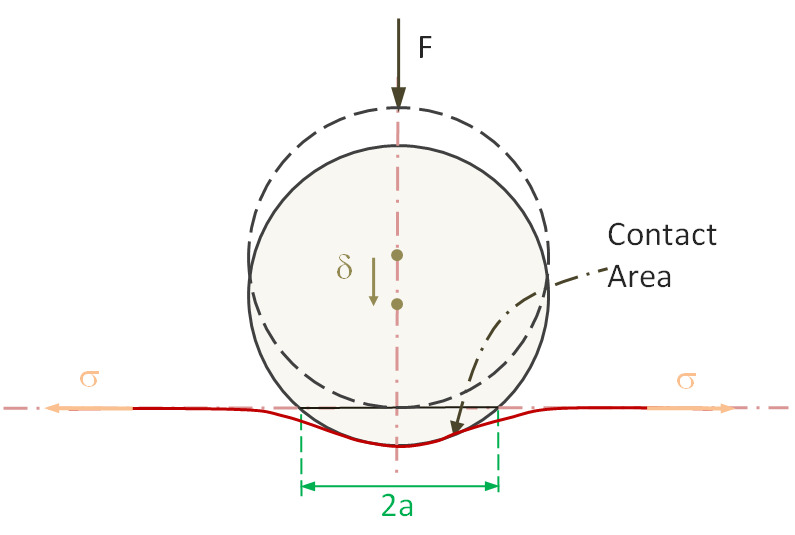
The theory of contact mechanics dates back to Heinrich Hertz (1881)1 who was the first to predict how the force induced contact area of two bodies of various geometries is effected by the force of indention. Hertz found that the radius of the circle of contact aH is a function of the indenter load F, the spherical indenter radius R, and the elastic properties of the contacting materials. His relation reads
aH3 = F · R* / E*
where R* is the equivalent radius given by
R* = R1 · R2 / (R1 + R2)
and E* is the equivalent elastic modulus of the indenter and the sample which could be two spherical bodies,
E* = (4/3) · {(1 - ν12) / E1 + (1 - ν22) / E2}-1
In the case of two indentical materials, the equation above simplifies to
E* = (2/3) · E / (1 - ν2)
and in the case of a stiff material in contact with a very soft material
E* ≈ (4/3) · E / (1 - ν2)
where E and ν are the Young’s modulus and the Poisson’s ratio, respectively. The Hertz theory has been shown to accurately describe the contact area between (large) elastic bodies with negligible adhesion forces. However, it is not applicable to soft elastic materials.
Rigid Sphere indented by a Vertical Force
Derjaguin-Muller-Toporov Theory
For small bodies where the surface-to-bulk ratio becomes significant or for soft matter like pressure sensitive adhesives, attractive interaction forces have to be included into the model as well. One such model is the model of Derjaguin, Muller and Toporov2 or short DMT model, which describes the deformation of bodies by including adhesion forces to the Hertz contact equation. In the case of two spherical bodies, the adhesion force can be described with the Bradley equation:
FA = 2π EA R*
where EA is the work of adhesion
EA = γ1 + γ2 - γ12
Combining the two forces gives the DMT equation:
F = E* aDMT3 / R* - 2π EA R*
or
aDMT3 = R*/ E* · (F + 2π EA R*)
Obviously, the two bodies separate only after applying a negative load, Fc,
Fc = - 2π EA R*
At this point, the contact radius is zero. Thus, Fc is the critical force required to separate the two bodies. The model also predicts the contact radius at zero load:
aDMT = (2π EA R*2 / E*)1/3
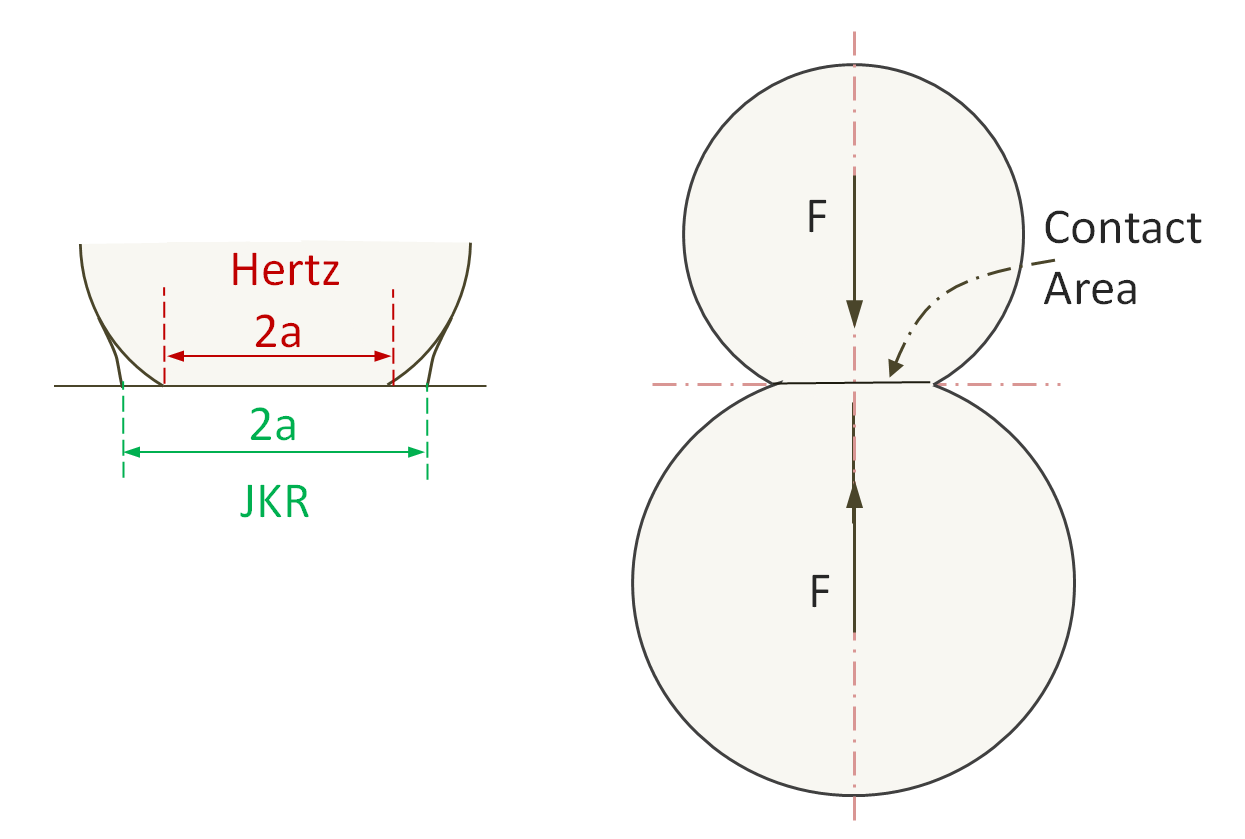
Johnson–Kendall–Roberts Theory
The Johnson–Kendall–Roberts (JKR) theory3 describes how two bodies adhere together and what deformation they undergo when in contact with each other. The deformation is the result of two opposing forces which results from surface tension and elastic deformation.
The JKR theory was originally developed to describe the macroscopic contact between a soft, adhesive material (tacky polymer) and a rigid material. However, it also has been widely applied to microscopic systems.
Johnson, Kendall, and Roberts (1971) assumed that the adhesive forces are only present in the contact region. The model is based on the concept of minimization of energies present in the deformation zone, i.e. it balances adhesion energy, which favours contact, against elastic energy, which opposes deformation.
Schematic of Contact Area
According to the JKR theory, the contact radius for a rigid sphere in contact with a soft elastic half space is given by
aJKR3 = R*/ E* · {F + 3π EA R* + [6π EA R*F + (3π EA R*)2]1/2}
where E is the equivalent elastic modulus and EA is the energy of adhesion. Upon application of a negative load, separation of the surfaces will occur when the external force equals
Fc = - (3/2)π EA R*
Thus, the pull-off force is independent of the elastic modulus, that is, it depends only on the equivalent radius of curvature and on the work of adhesion. The value for the critical contact radius at zero load is given by
aJKR = (6π EA R*2 / E*)1/3
For the special case of a soft elastic half space in contact with a rigid plane, the JKR formula can be written as
aJKR3 = 3(1 - ν2) R / (4E) · {F + 3π EA R + [6π EA RF + (3π EA R)2]1/2}
The value for the critical contact radius at zero load is given by
aJKR = [9(1 - ν2) π EA R2 / E]1/3
Contact Width Versus Indenter Force
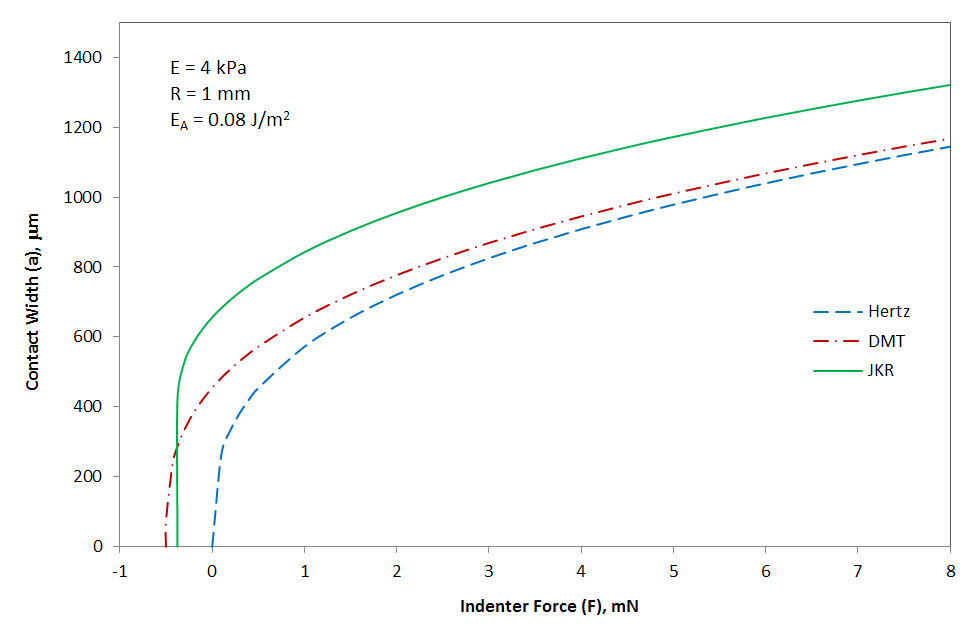
The figure above shows the contact area as a function of applied force for a polymer sphere of 1mm radius and of low Young's modulus on a flat, stiff substrate with much larger modulus. This situation can be decribed with the JKR model whereas the Hertzian and the DMT model predict too small contact areas. The JKR curve shows a relative large zero force contact width of about 650 μm. From such a curve, both the youngs modulus E and the energy of adhesion EA can be deduced. The curves also show what happens when the sphere is pulled away from the surface and the force becomes negative. The sphere suddenly breaks contact when the critical value Fc is reached:
Fc = - (3/2) π EA R
Beyond DMT and JKR Adhesion
There are many cases where adhesive forces well above one Newton
are encountered. In some cases the forces that are required to
separate materials
are a hundred to thousand times greater than what the DMT and JFK
theory predicts. To understand what causes these strong
interactions, we have to understand the energy dissipation
processes in materials during separation. Obviously, other forces
than pure surface tension induced adhesion must be present
to achieve fracture energies in the range of 100 to 1000 J/m2. This can be easily proven; if no other forces than surface tension is present, then the total energy
per unit area to produce a new interface is EA ≈ 2 γ. For a van der Waals liquid, the surface tension is in the range of γ = kT / l2,
where l is the size of a molecule, T is the absolute temperature and k is the Boltzmann constant.
This gives values in the range of EA ≈ 0.1 J/m2 which is much smaller than
the typical fracture energy of a pressure sensitive adhesive.
Obviously, a van-der-Waals liquid is a poor adhesive.
The strength
can be improved by creating stronger interactions between the molecules of the polymers and between the polymers and the substrate. Particular strong interactions are covalent
and ionic bonds, but hydrogen bonds will also lead to considerable improved adhesion.
The typical bond energy of a covalent bond is in the range of Ub = 1 eV
or 1.6 x 10-19J. Assuming one bond per molecule, vb ≈ 1/(0.3x10-9) = 1 x 1019 m-2,
the corresponding fracture
energy, G = vbUb, is in the range of a few J/m2. This
is a significant improvement but still far below the observed bond
strength of adhesive joints. Thus, the nature of adhesion cannot be
explained alone by surface tension and rupture of chemical and
physical bonds.
The true reason for the observed high rupture energies is strong plastic deformation of the materials. In the case of two polymers, this can be achieved even without strong bonds between the two materials. Let us consider a smooth plastic-plastic interface; one of the plastics may or may not be crosslinked whereas the second plastic consists of linear polymer chains. If the two polymeric materials are soluble in each other and if the chains are mobile enough, some chains may interdiffuse, that is, mixing of the two plastics at the interface may take place until the interface vanishes. This phenomenon is known as interdiffusion or interpenetration. Even modest “intermingling” of the polymers of the two substrates can lead to a noticeable increase in adhesion and if the polymers are truely entangled when they cross the interface, strong adhesion will occur due to physical crosslinking (entanglement). The strong plastic deformation during fracture leads to much larger fracture energies.
The high fracture energies can be only understood as simulataneous stretching and disentanglement of many polymer chains that are all interconnected, i.e. that form a network. Take, for example, a rubber band. The force that is required to stretch it to its breaking point is relative small, but when we tie a large number of them together, say N, then the energy that is required to snap them is N times the energy of one rubber band because all rubber bands are stretched simultaneously. This is exactly what happens when an elastomeric material is in contact with an amorphous plastic and some intermingling of polymer chains at the interface occurs.
Since both polymer interpenetration and polymer disentanglement during fracture are dynamic processes, i.e. they are not instantaneous, the bond strength will depend on the contact time, the applied load (or pull speed), and the temperature.
The theory of crack propagation and energy dissipation through friction has been first described by Raphael and de Gennes4,5.
References and Notes
Heinrich Hertz, J. Reine Angew. Math. 92, 156 (1881)
B. V. Derjaguin, V. M. Muller and Y. P. Toporov, J. Colloid Interface Sci.53, 314 (1975).
K. L. Johnson, K. Kendall and A. D. Roberts, Proc. Roy. Soc. London A 324, 301 (1971)
E. Raphael, P.G. de Gennes, J. Phys. Chem. 96, 4002 (1992)
Let us assume two chemically identical materials, one is crosslinked and the other consists of linear chains that are able to cross the interface. According to de Gennes, the total work of adhesion at zero velocity for relative short polymer portions crossing the interface can be described by following simple relationship G0 ≈ EA + Σ N U where Σ is the number of chains per unit area that cross the interface (connectors), N is the number of monomers in the "connector" subchains, and U is the energy needed for a repeat unit to slip past another.
N.K. Myshkin and A.V. Kovalev, Polymer Tribology, pp. 3-37 (2009)