Redox Free-Radical Polymerization
Free-radical polymerization (FRP) techniques are of great importance in the polymer industry and are utilized in a broad range of everyday products including coatings, inks, paints, composites, adhesives, and dental products.1 A FRP can be triggered by a number of methods including heat, chemical reactions as well as by electron beam and UV radiation. Typically, the most energy efficient initiation methods are based on bimolecular redox reactions, which can be carried out under mild conditions and without any additional energy source.2
One of the oldest redox system for the generation of free radicals is Fenton’s reagent, (Fe2+/ H2O2):3,4
Fe2+ + HOOH → HO• + HO- + Fe3+
In this reaction, precisely one electron is transferred from the iron (II) ion to the peroxide, triggering its dissociation and the formation of an iron (III) ion.
Metal cations can also promote the decomposition of a number of other peroxides, including alkyl and acyl peroxides:
Fe2+ + ROOR → RO• + RO- + Fe3+
where R is an alkyl group or a hydrogen atom H. The alkoxy and hydroxy fragment with an unpaired electron are called free radical initiators. Following their generation, some of them react with a monomer thereby creating a new radical that can start a chain growth polymerization:
RO• + (n+1) M → ROMnM•
A major drawback of Fenton's redox system is that the reducing metal will also reduce radicals which inhibits the reaction. Furthermore, alkoxy and hydroxy radicals may also react with hydroperoxides in order to form inactive peroxyl radicals:5
Fe2+ + RO• → Fe3+ + RO-
HOOH + RO• → ROO• + H2O
To increase the efficiency, the metal ions need to be quickly reduced, which can be achieved with organic reducing agent such as vitamin C.
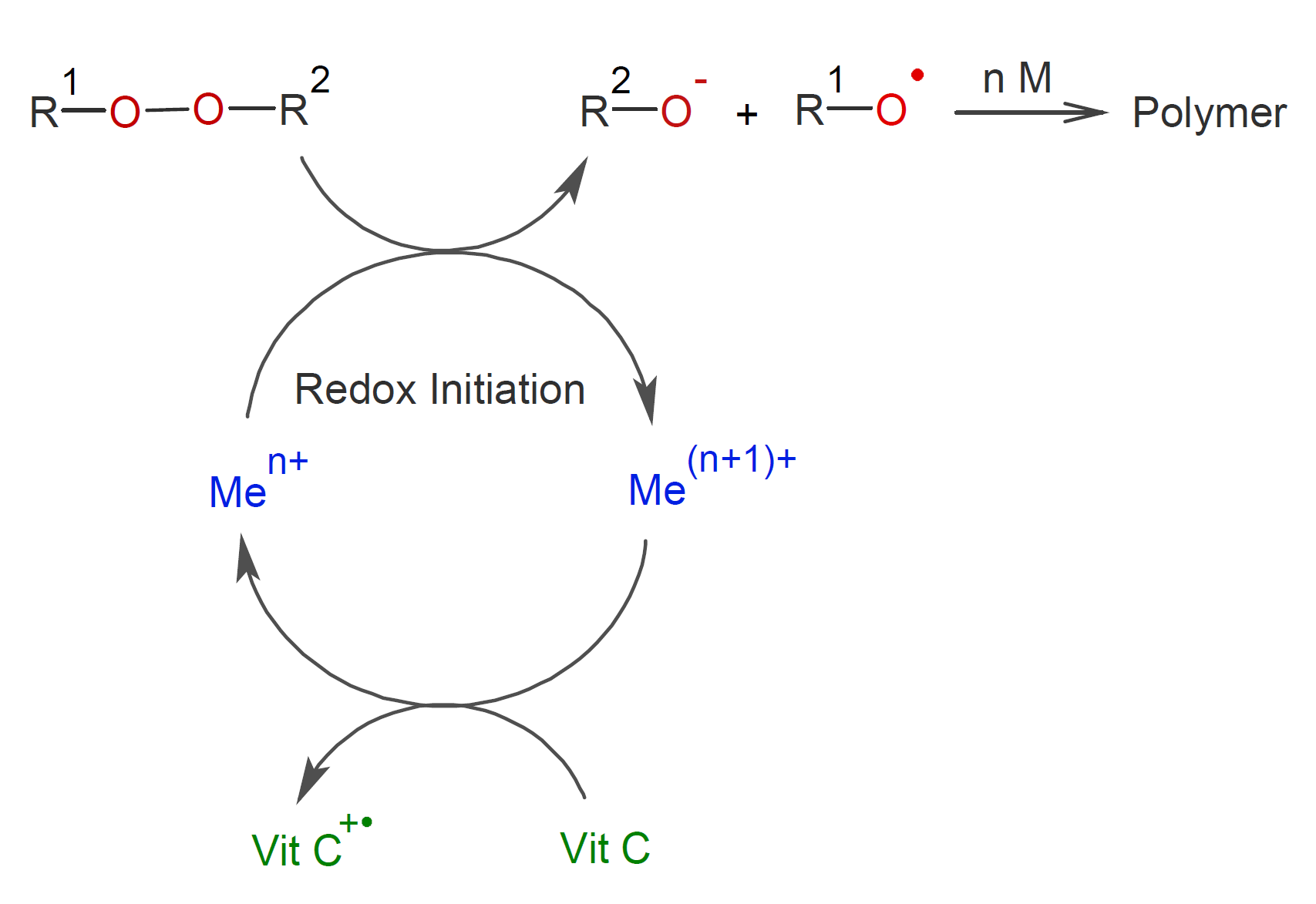
Two other highly efficient redox system are disulfides and persulfates in combination with metal reducing agents:5,6
Fe2+ + RS−SR → Fe3+ + RS• + RS-
Fe2+ + -O3S−O−O−SO3- → Fe3+ + -O3SO• + SO42-
Both redox systems can undergo many side reactions. For persulfates, for example, following additional redox reaction have been reported:5
H2O + -O3S−O−O−SO3- → 2 HSO4- + H2O2
H2O2 + Fe2+ → •OH + - OH + Fe3+
-O3SO• + Fe2+ → Fe3+ + SO42-
The persulfate redox system is one of the most frequently employed intiator systems in aqueous (emulsion) polymerization of water-soluble monomers. It is very efficient because both the •OH and O3SO• radical can initiate polymerization.
One of the most popular redox systems for polymerization in non-aqueous media is dibenzoyl peroxide (BPO) / tertiary aromatic amine such as N,N-dimethylaniline which considerably accelerates the decomposition of BPO. This redox system has been studied in great detail.7-10 It was postulated that the tertiary amine and BPO form a cyclic transition molecular complex which decomposes into an ion pair and a benzoyl radical. The subsequent transfer of a proton (H+) produces an additional amino alkyl radical. Both the amino alkyl radical and the benzoyl radical are efficient initiators for free radical polymerization.
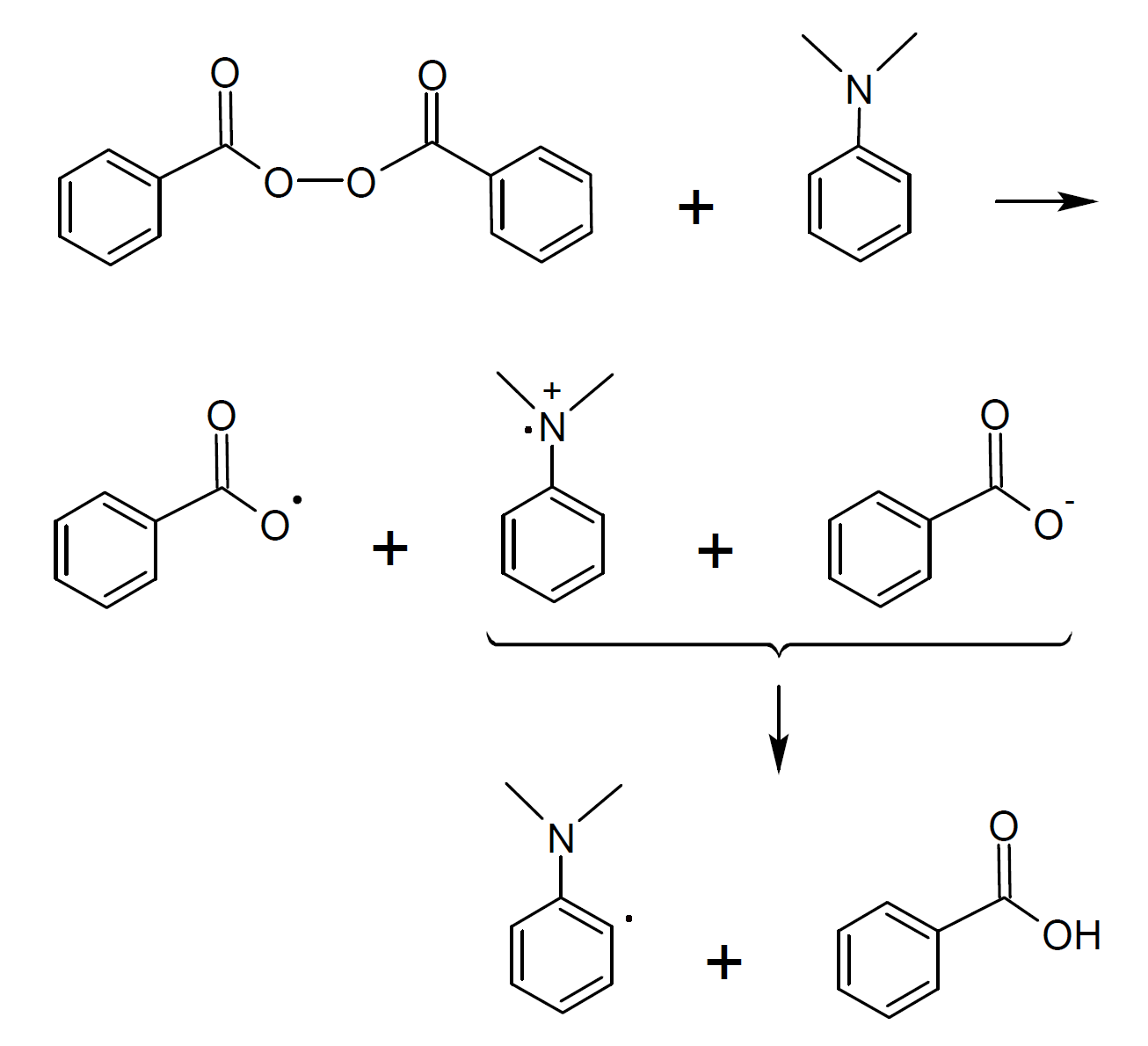
Other peroxides such as diacyl peroxides and hydroperoxides are also susceptible to this type of redox reaction. The activity of these system can be further enhanced by the addition of a soluble metal salt such as metal (Cu, Mn, V, Co) acetyl acetonates.
Another redox initator system described in the literature is based on organoborane compounds such as triethylborane which releases radicals when exposed to oxygen. This initiator system can be employed to initiate polymerization of a large number of vinyl monomers at ambient conditions including vinyl chloride, propylene, isobutylene and (meth)acrylates.12 One proposed mechanism of initation is based on diffusion-controlled oxidation of the trialkylborane:12-14
R3B + O2 → R2BO2• + R•
R• + O2 → RO2•
RO2• + R3B → R2BOOR + R•
An alternate oxidation mechanism is based on oxygen insertion between the boron-carbon bond, generating directly a boron-containing peroxide:13
R3B + O2 → R2BOOR
R2BOOR → R2BO• + RO•
Thus, no intermediate radical is produced. Quantum chemical calculations suggest that this is the preferred mechanism of borane oxidation.14-16
Over the years, many other highly reactive redox system have been discovered.11, 18-23 Most of these are based on metal acetyl acetonates and various compounds with labile hydrogen. Some of these initiator systems possess excellent stability, low toxicity, and tunable gel time.
References & Notes
Peter Nesvadba, Radical Polymerization in Industry, in Encyclopedia of Radicals in Chemistry, Biology and Materials, John Wiley & Sons 2012
Mild conditions for redox polymerization means room temperature, no purification of the monomers, and no additional energy source.
H.J.H. Fenton, J. Chem. Soc., Trans., LXXIII, Vol. 65, 899–910 (1894)
Dietrich Braun, Origins and Development of Initiation of Free Radical Polymerization Processes, Int. J. of Poly. Sci. Vol. 2009, e893234/1-10
A.S. Sarac, Prog. Polym. Sci., 24, 1149–1204 (1999)
P. Garra, C. Dietlin, F. Morlet-Savary, F. Dumur, D. Gigmes, J.P. Fouassier, J. Laleve, Progr. Poly. Sci., 94, 33-56 (2019
B. Vazquez, C. Elvira, J.S. Roman, B. Levenfeld, Polymer, Vol. 38 (17) 4365-4372 (1997)
I.D. Sideridou, D.S. Achilias, O. Karava, Macromolecules, 39, 6, 2072-2080 (2006)
T. Sato, S. Kita, T. Otsu, Makromol. Chem., 176 (3), 561-571 (1975)
T. T. Vasil'eva, and R. Kh. Freidlina, Russ. Chem. Bull., 17, 1039 - 1041 (1968)
P. Garra, C. Dietlin, F. Morlet-Savary, F. Dumur, D. Gigmes, J.P. Fousassier, J. Lalevee, Prog. Polym. Sci., 94, 33-56 (2019)
M. Mishra and Y. Yagci, Handbook of vinyl Polymers, CRC Press, 2009
P.B. Brindles, R.G. Pearson, J. Polym. Sci., Part B: Polymer Letters, 6, 831-835 (1968)
M.F. Sonnenschein et al., Macromolecules 37, 7974-7978 (2004)
H.C. Brown, M.M. Midland, J. Chem. Soc. D: Chem Commun. , 699-700 (1971)
F.J. Welch, J. Polymer. Sci., 61 (171), 243-252 (1962)
Note, organoborane compounds are highly flammable when exposed to air. To reduce their pyrophoricity, amines are often added, which form a complex with the borane. To initiate polymerization with these complexes, the borane has to be liberated first, for example by salting the amine with a Bronsted or Lewis acid.
P. Garra, F. Dumur, M. Nechab, F. Morlet-Savary, C. Dietlin, B. Graff, E.P. Doronina, V.F. Sidorkin, D. Gigmes, J.P. Fouassier, J. Lalevée, Macromolecules, 51, 16, 6395-6404 (2018)
A. Arar, H. Mokbel, F. Dumur and J. Lalevée, Molecules, 25, 1602 (2020)
P. Garra, F. Dumur, M. Nechab, F Morlet-Savary, C. Dietlin, B. Graff, D. Gigmes, J.-P. Fouassiera, J. Lalevee, Polym. Chem., 9, 2173-2182 (2018)
C.W. Boeder, Surface Activator For Redox-Initiated Adhesives, US Patent 5,003,016 (1989)
P. Garra, F. Dumur, F. Morlet-Savary, C. Dietlin, D. Gigmes, J.P. Fouassier, J. Lalevee, J. Polym. Sci., Part A: Polym Chem., 55, 3646-3655 (2017)
M.T. Plaumann, M. Danebrock, Polymerisation Initiator System for Dental Resins, DE19757277A1 (1997)
April 20, 2020 & Revised July 28, 2020