Synthesis of Polyamides
Polyamides are one of the most important and most widely used classes of polymers. They are macromolecular compounds having amide groups in the polymer backbone which form strong interchain hydrogen bonds that are responsible for the high strength and thermal stability of polyamides. This class of polymers can be synthesized by various methods including step-growth polycondensation, ring-opening polymerization, and polyaddition reactions using a large number of monomers. This allows for the synthesis of polyamides with tailored properties and diverse structure that can be readily processed into films, fibers and molded products.
The largest volume polyamide is poly(hexamethylene adipamide), which is produced by polycondensation of hexamethylene diamine with adipic acid. This polymer is known as Nylon 6,6 and was first synthesized by Wallace Carothers in 1935.1
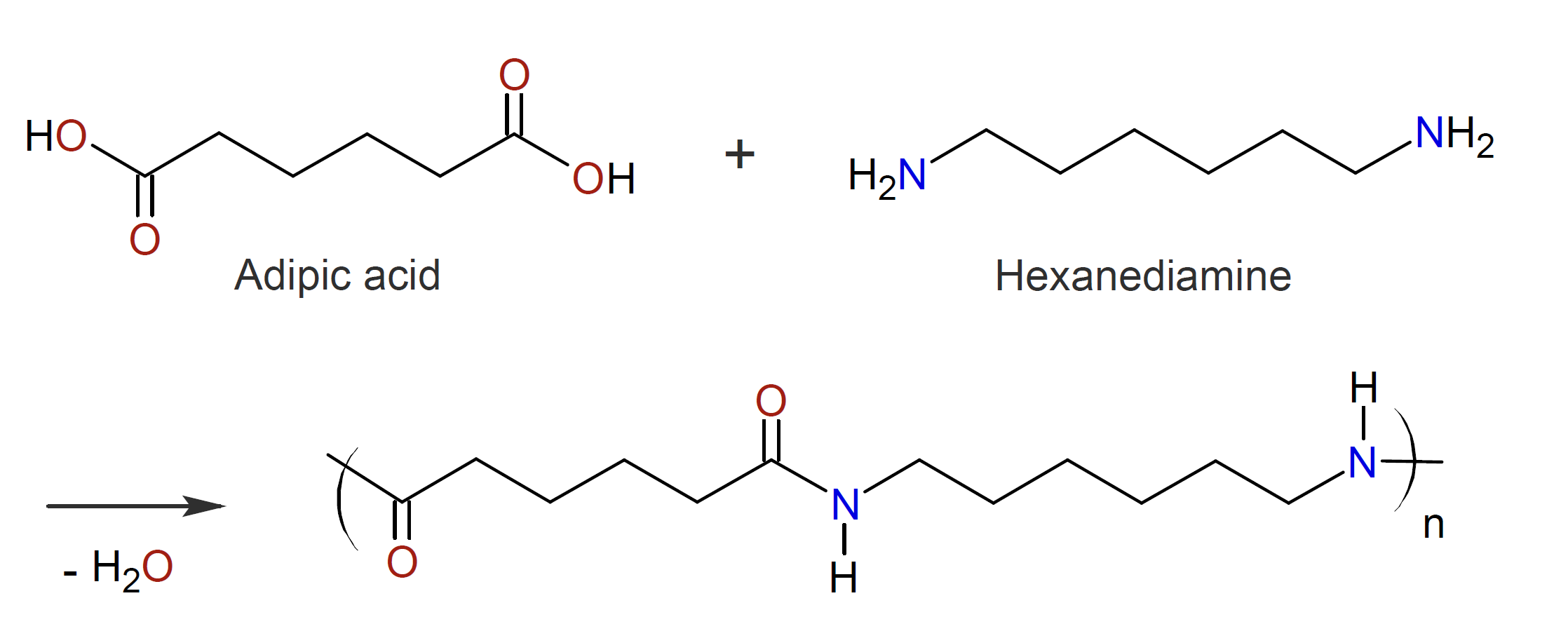
Often the two reactants are converted to a “Nylon salt” prior polymerization.2 These salts contain substantially equivalent amounts of diamine and dicarboxylic acid. The use of Nylon salt is advantageous, since it ensures equivalency of amine and acid reactants. It also eliminates impurities present in the original damine and dibasic acid which are difficult to remove by other means.
Unlike polyesterification, polyamidation of aliphatic acids and amines does not require a strong acid catalyst because polyamidations are exothermic reactions with much higher reaction rates.1 Furthermore, the equilibrium of polyamidation is more shifted towards the condensation product. However, the condensed water should still be removed, for example, by using a vacuum, since water could be detrimental to the forming process and to the performance of the polyamide.
In place of a diamine and dicarboxylic acid (or its salt), a diamine and a diacid chloride may also be used in the preparation of polyamides (Schotten-Baumann reaction).3,16 The reaction may be carried out in the presence of a solvent or diluent (which is not a solvent for the polymer) or in the melt state. A catalyst may be employed to accelerate the reaction such as triphenylphosphine (Ph3P), pyridine and halide.4
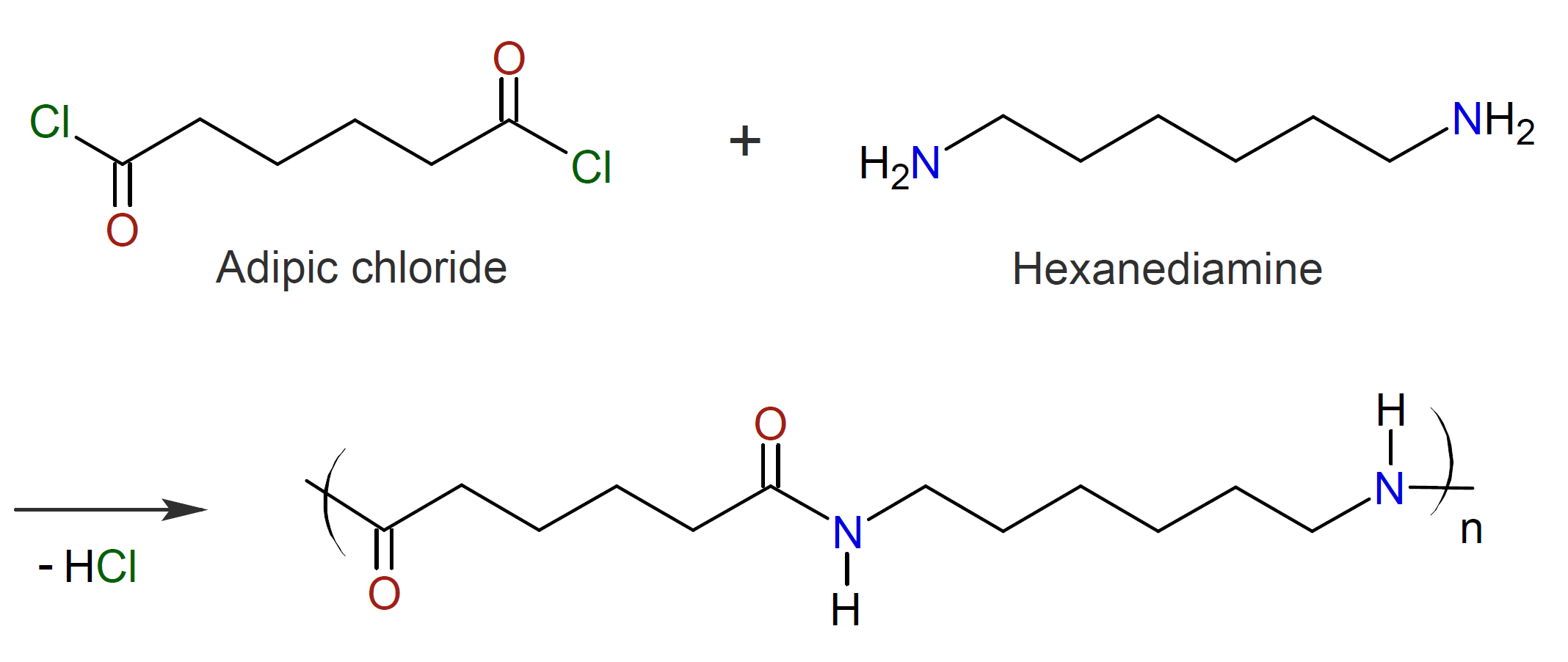
This reaction is very fast, irreversible and can be started at room temperature.16 A drawback of this method is the formation of hydrochloric acid (HCl) as a byproduct which is difficult to remove. For this reason, an inorganic base (NaOH) is added to the aqueous phase to neutralize this by-product (acid acceptor) which otherwise could negatively affect the polymerization.
The method can also be employed to synthesize fully aromatic polyamides, known as aramids. However, due to their high melting point, they are typically prepared in solution at relative low temperature or, alternatively, by direct condensation of an aromatic diacid with a diamine at high temperature.6,7 Suitable solvents include polar aprotic amide compounds such as hexamethylphosphoramide (HMPA), N,N-dimethylformamide(DMF) and N,N-dimethylacetamide (DMA).7 The two best known aromatic amides synthesized by these methods are poly(p-phenylene terephthalamide), called Kevlar, and poly(m-phenylene isophthalamide), known as Nomex, which were discovered by S.L. Kwolek and P.W. Morgan (DuPont) in 1958 and later commercialized in 1961 and 1971.8-10 Their synthesis and chemical structure is shown below.
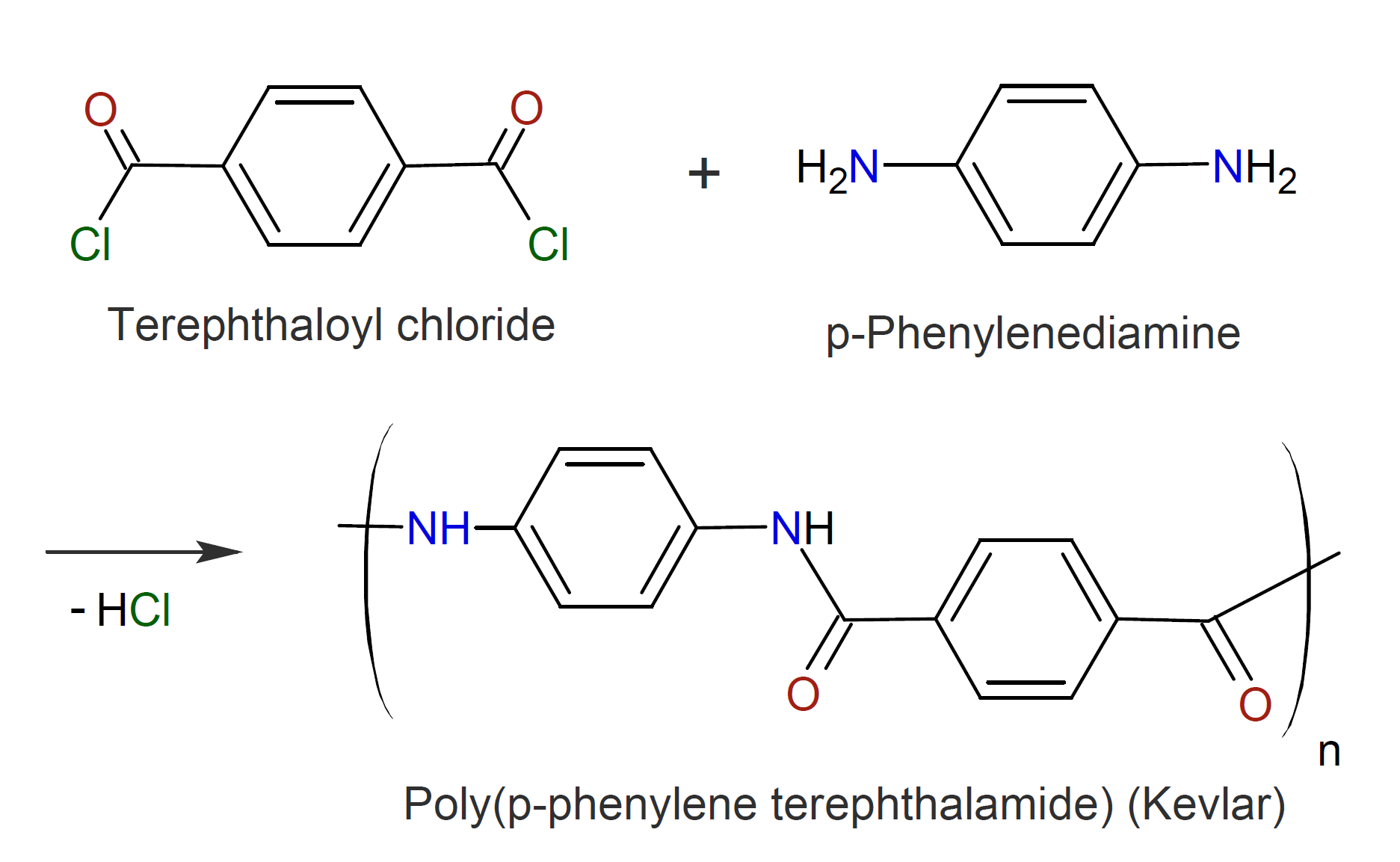
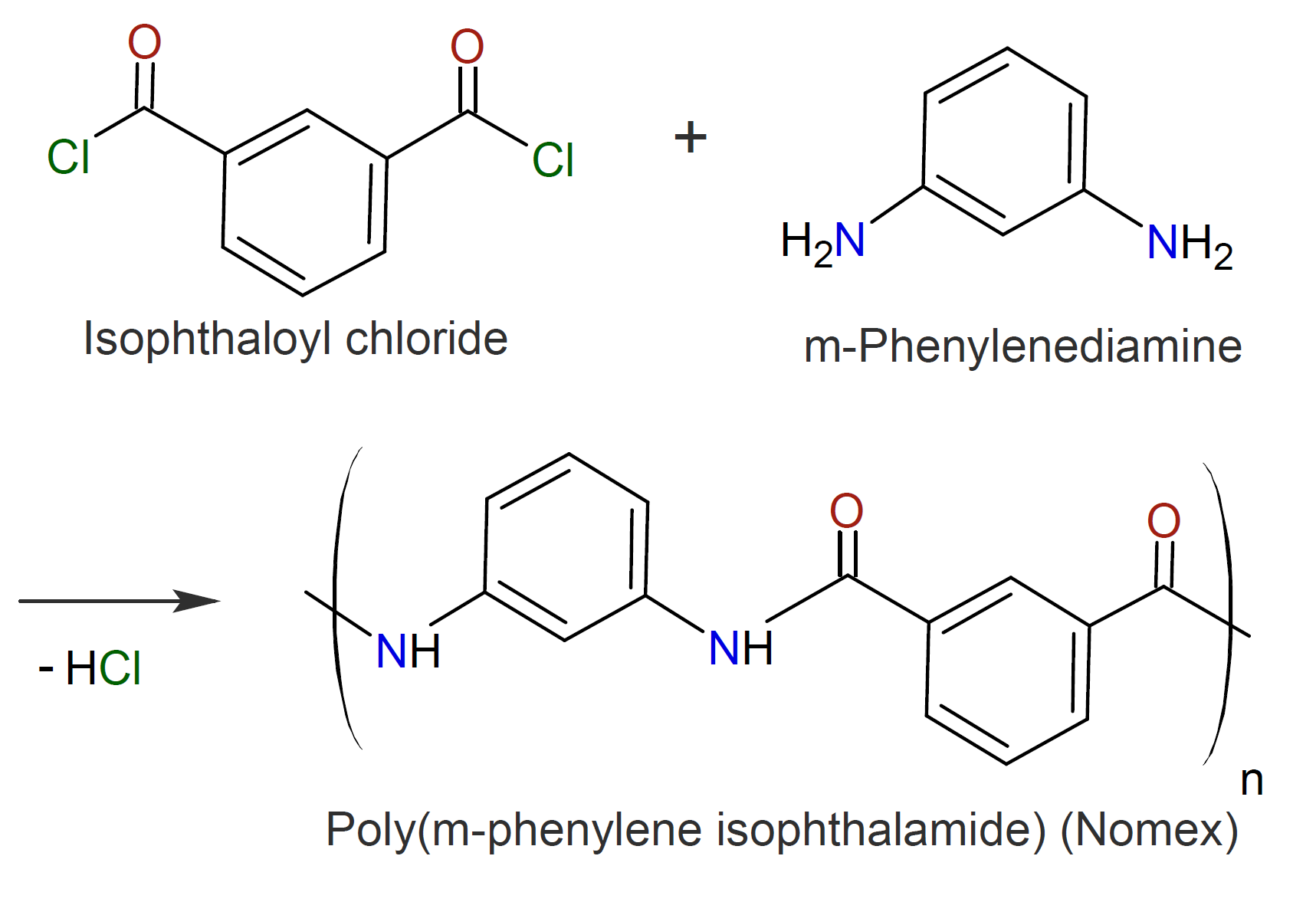
Acid chlorides and amines have also been employed in interfacial polymerization in which the diamine monomer is dissolved in an aqueous phase and the diacid chloride in an organic solvent often in the presence of a salt such as LiCl and/or CaCl2 which decreases the strength of interchain hydrogen bonds and thus the tendency of the polymers to crystallize.11,16 However, the reaction at the interface is difficult to control because the instantaneous concentration of the reactants depends on their (slow) diffusion rate which can be a limiting factor in the polymerization process. In addition, precipitation of the growing polymers produces aramids with a broad molecular mass distribution, which is detrimental to the properties of aramid fibers and films. To improve the polymerization process, the acid chloride and diamine are first slowly reacted in a solution without an acid acceptor. The mixture is then added to an aqueous solution containing an acid acceptor and stirred vigorously to promote interfacial polymerization. This process produces polyamides in high yield in a short period of time and under ambient condition5 with molecular weights (much) higher than those of aramids produced by solution polymerization techniques which results in better film and fiber properties.7
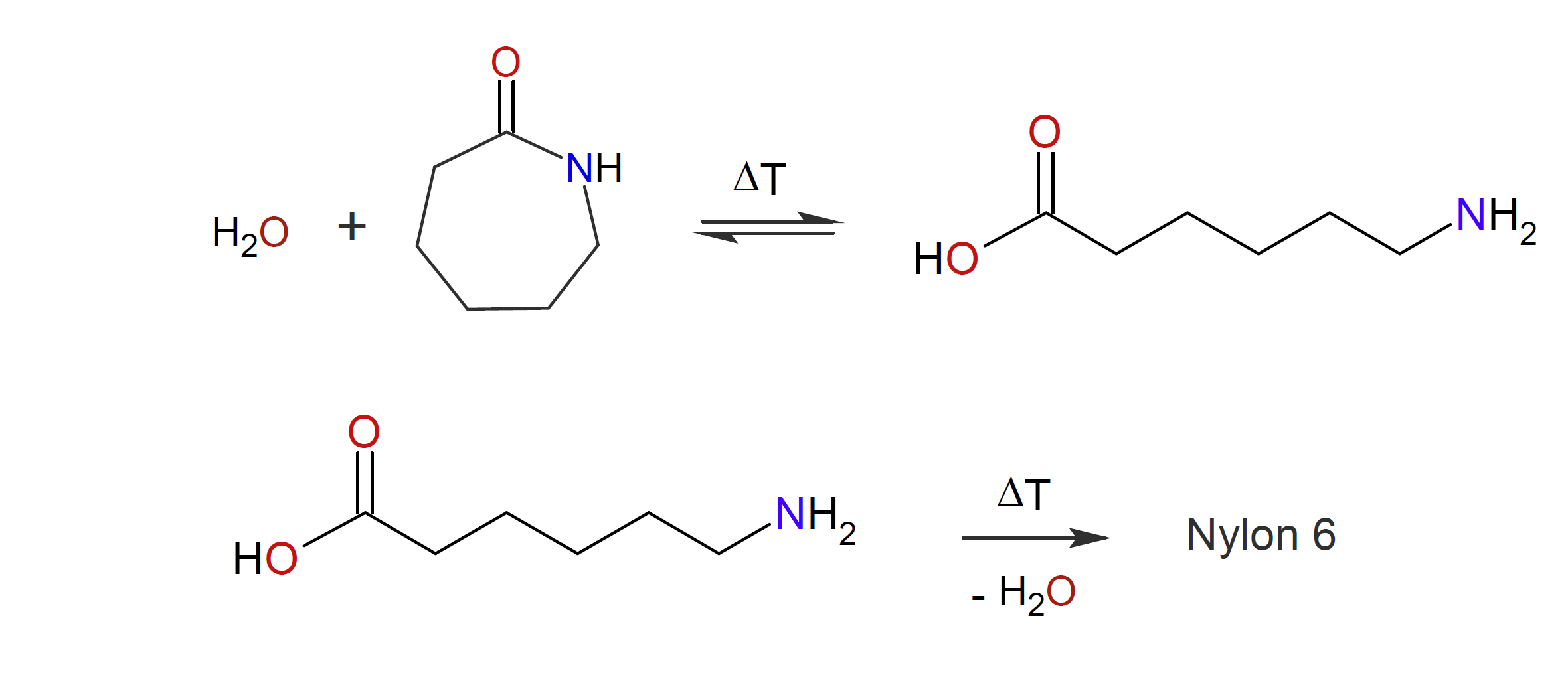
A third method that yields polyamides is ring-opening polymerization of lactams which can be achieved in two different ways:
a) Ring-opening by hydrolysis of lactams by water into an amino acid with simultaneous or subsequent
polycondensation:12,13
b) Anionic ring-opening with subsequent addition to another lactam (AROP):14, 15
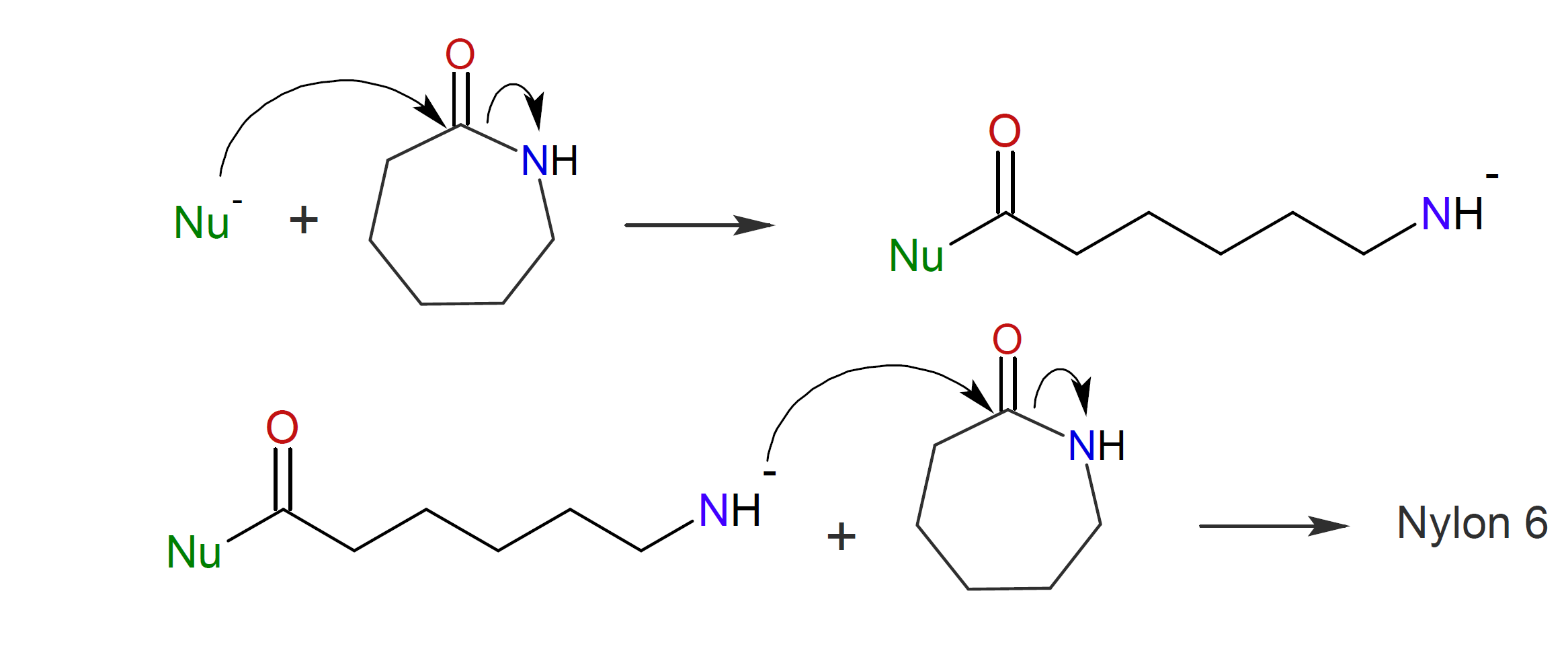
The polymerization can be initiated by a large number of anionic initiators including carbanions, alcoholates, silanoates, carboxylates, thiolates, alkoxides, and tertiary amines. The most important polyamide manufactuered by either method is polycaprolactam also known as Nylon 6. This polymer was first discovered by Paul Schlack in 193914 when working for I. G. Farbenindustrie, back then, the largest chemical company in the world. A year later, William Hanford12 discovered an alternate method to synthesize the same polymer when working for du Pont.
References
Wallace Hume Carothers, E.I. Du Pont de Nemours & Company, Synthetic Fibers, United States Patent 2,130,948 (1937)
Wallace Hume Carothers, E.I. Du Pont de Nemours & Company, Diamine-Dicarboxylic Acid Salts and Process of Preparing Same, United States Patent 2,130,947 (1938)
Herbert Blades, High Strength Polyamide Fibers and Films, United States Patent 3,869,429, E.I. du Pont de Nemours and Company (1975
N. Ogata, K. Sanui, and S. Tan, Polymer Journal, Vol. 16, No. 7, pp 569-574 (1984)
Mohammad Asif Ali and Tatsuo Kaneko, Polyamide Synthesis in Encyclopedia of Polymeric Nanomaterials, Springer Berlin 2015
N. Yamazaki, F. Higashi, and J. Kawabata, J. Polym. Sci. Polym. Chem. Ed, 12, 2149, (1974)
M. Trigo-Lopez, J.M. Garcia et al., Aromatic Polyamides in Encyclopedia of Polymer Science and Technology, John Wiley & Sons 2018
Stephanie L. Kwolek and Paul W. Morgan, E.I. Du Pont de Nemours & Company, Process of Making Wholly Aromatic Polyamides, United States Patent 3,063,966 (1962)
Stephanie L. Kwolek, E.I. Du Pont de Nemours & Company, Process for thr Production of Highly Orientable, Crystallizable, Filament-Forming Polyamide, United States Patent 3,287,323 (1966)
L.F. Beste and C.W. Stephens, E.I. Du Pont de Nemours & Company, Composition Comprising a Synthetic Linear Polymer, Organic Solvent and Inorganic Salt, United States Patent 3,068,188 (1962)
Leo Vollbracht, Akzo N.V., Process for the Preparation of Poly(p-phenylene terepthaltamide, United States Patent 4,308,374 (1981)
William E. Hanford, E.I. Du Pont de Nemours & Company, Process for Preparing Polyamides from Cyclic Amides, United States Patent 2,241,322 (1941)
Patrick V. Papero and Orvill E. Snider, Allied Chemical Corp., Caprolactam Polymerization, United States Patent 3,090,773A (1963)
Paul Schlack, I.G. Farbenindustrie Aktiengesellschaft, Preparation of Polyamides, United States Patent 2,241,321 (1941)
Edward H. Mottus, Ross M. Hedrick and John M. Butler, Monsanto Chemical Company, Preparation of Polycaprolactam using N-acyl Activators, United States Patent 3,017,391 (1962)
E.I. Wittbecker and P.W. Morgan, J. Polym. Sci., 40, 289-297 (1959)
September 6, 2020