Synthesis of Linear Saturated Polyesters
Polyesters are one of the most important and widely used classes of polymers. They are macromolecular compounds having ester groups in the polymer backbone instead of ester groups in the side chain, as in the case of poly(vinyl acetate) and poly(methyl methacrylate). This class of polymers can be synthesized from a large number of compounds using a variety of methods including step-growth polycondensation, ring-opening polymerization, and polyaddition reactions.
Some polyesterification reactions of polyols with diacids and their derivaitves are shown in the scheme below. A very common method is transesterification of a dialkyl ester (scheme d).1 This method is often the method of choice for polymers with high melting point and dicarboxylic acids with low solubility.2 Another common method is direct esterification of a dicarboxylic acid with a diol3 (scheme f). This type of polyesterification is preferably carried out at the boiling point of the mixture and has a higher reaction rate than transesterification.3
Polyesterifications are typically very slow and, therefore, have to be conducted at relative high reaction temperatures (> 200°C) and in the presence of a catalyst such as antimony, germanium, titanium, and aluminum compounds to achieve reasonable rates of reaction and high molecular weights.1-5 Despite the use of a catalyst, the overall reaction time is very long because the elementary growth step in polyesterification and polytransesterification is an equilibrium reaction and therefore, an increase in molecular weight can only be achieved if the produced alcohol or water is efficiently and continuously removed from the reaction vessel. However, this can be difficult during the later stages of polymerization due to the high viscosity of the resulting polymers. For this reason, polycondensations are often carried out in solution and at low pressure where the evaporating solvent may form an azeotropic mixture with the water or alcohol.
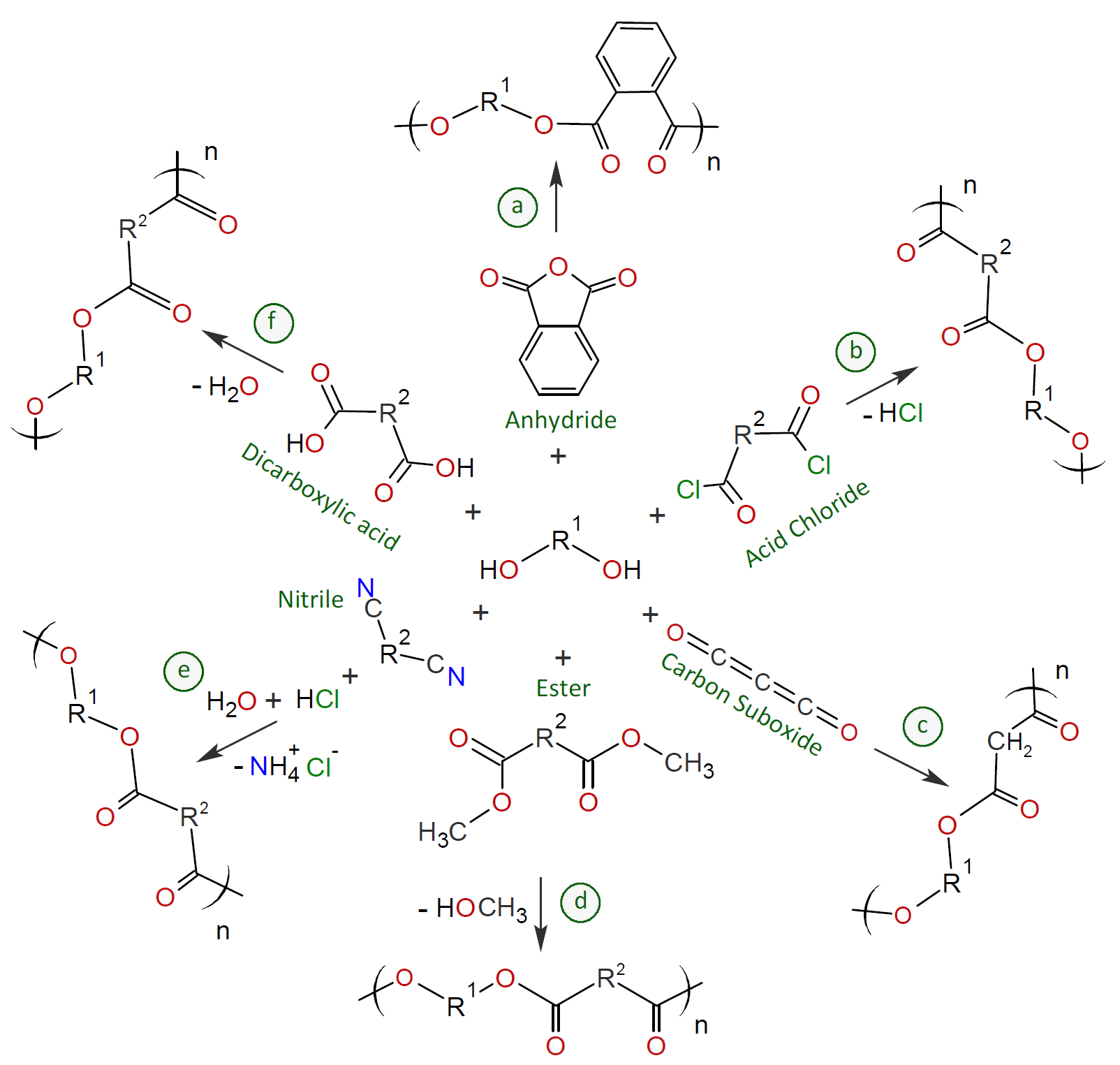
Polyols can be condensed with a number of other compounds to produce a variety of polyesters. However, the majority of these reactions have gained little to no commercial uses. For example, dibasic acid chlorides can be condensed with polyhydric glycols which yields hydrochloric acid as a by-product (scheme b).6 This reaction is non-reversible and often quite efficient when acid scavengers such as tertiary amines are added.2 Thus, the polymerization can be conducted without a high vacuum or without blowing inert gas for prolonged periods to achieve the removal of the by-product. Furthermore, the polymerization can be conducted at ambient temperature,7 whereas transesterification and direct esterification require very high reaction temperatures well over 200°C to achieve high degrees of conversion.3,4 On the downside, polycondensation of diols with acid chlorides is limited by the high cost of the dichlorides, and their sensitivity to hydrolysis and side reactions. Another reported non-reversible method is condensation of nitriles with glycols (scheme e).8 This reaction can be carried out at ambient as well.
Alternatively, polyesters can be synthesized from a single reactant, the so-called hydroxy acids. For example, p-hydroxyethoxybenzoic acid self-condenses in the presence of a catalytic amount of a metal catalyst such as dibutyl tin diacetate to following semi-aromatic polyester:
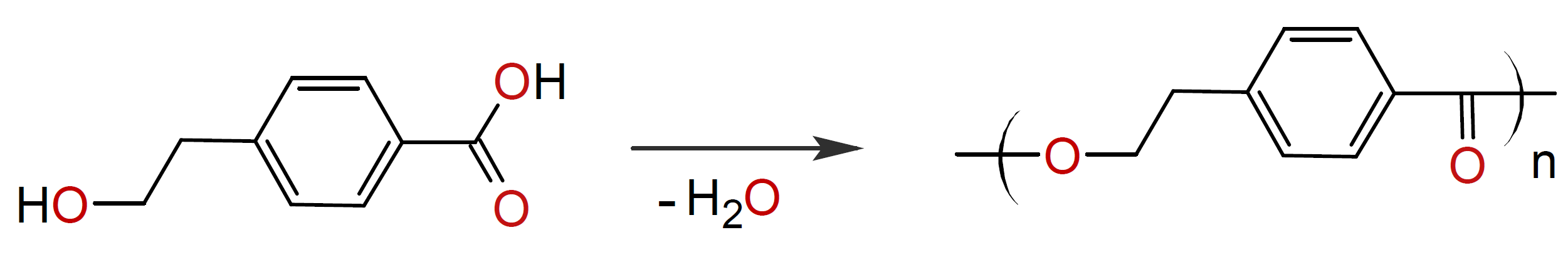
whereas self-condensation of 4-hydroxybenzoic acid and 6-hydroxy-2-naphthoic acid produces fully aromatic esters. However, direct self-condensation requires relative high reaction temperatures (> 300°C).9
Another polymerization method that produces polyesters but does not involve glycols is ester interchange between a dicarboxylic acid and a diester.2,10 For example, the reaction between glycol acetate and a terephthalic acid yields
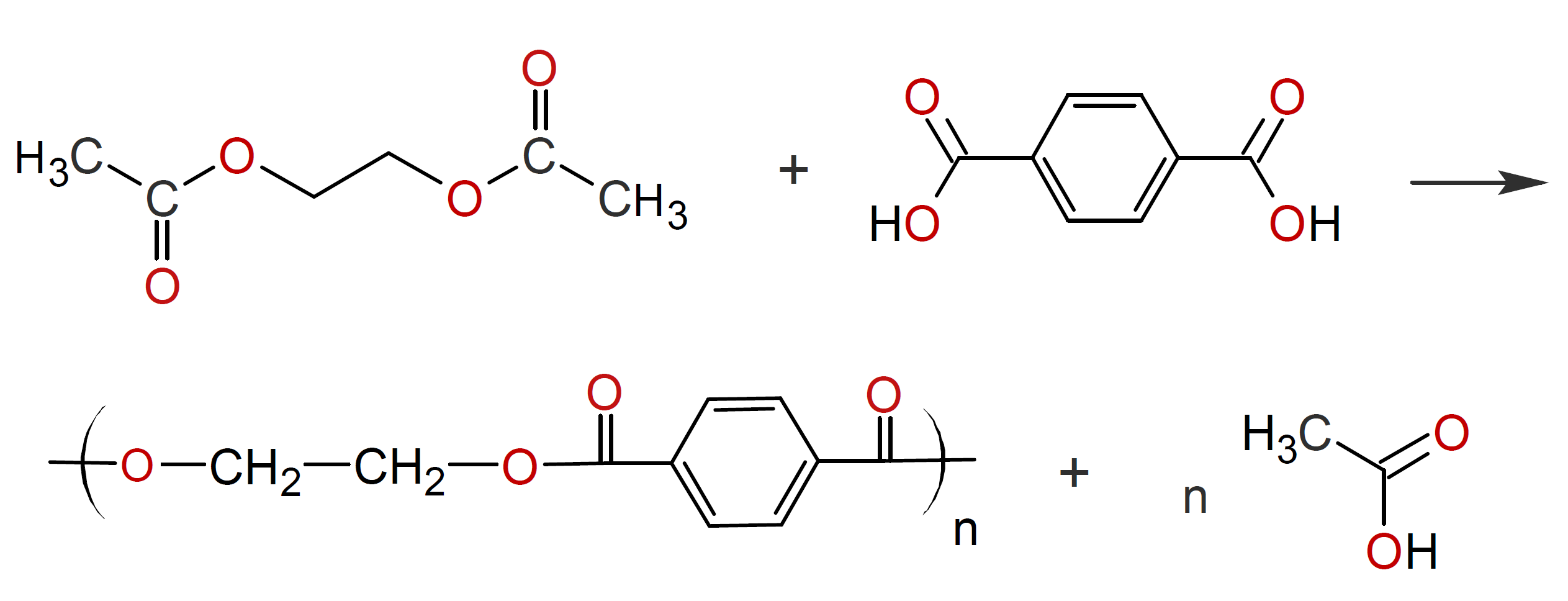
which is known as polyethylene terephthalate (PET) or ''polyester''.
Polyesters can also be prepared by polyaddition reactions. An advantage of addition polymerization is that no by-products are formed; in other words, there is no need for vacuum removal of low molecular weight compounds such as methanol or water and in many cases the reactions can be conducted at milder conditions.11 Two examples are the reactions of a polyol with a dicarboxylic acid anhydride (scheme a) and with carbon suboxide (C3O2).11 The latter yields a polymalonate (scheme c). There are numerous other addition reactions that yield polyesters but do not involve glycols. One such reaction is the addition of a epoxide to a diacid or acid anhydride:11, 12
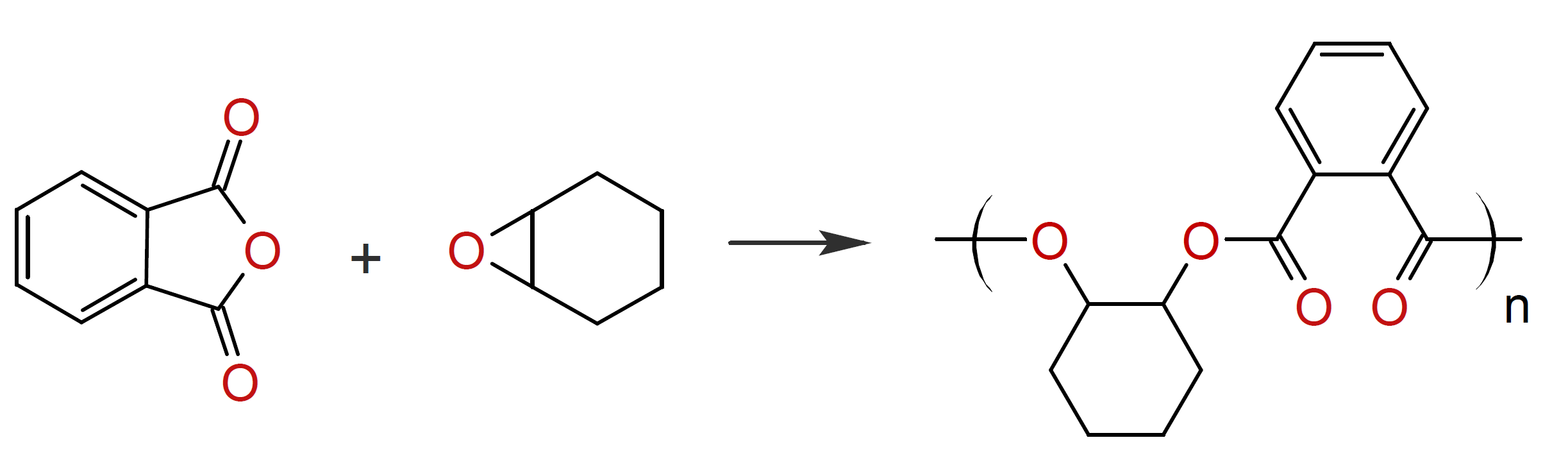
This reaction is catalyzed by amines, quaternary ammonium, antimony trioxide, or antimony pentachloride. Similar polyesters can be prepared by the reaction of a dicarboxylic acid with a cyclic carbonate:2
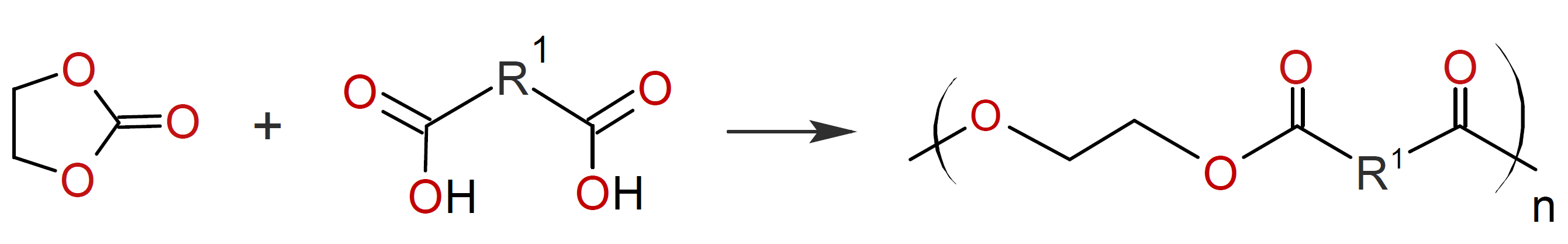
However, the occurrence of numerous side reactions during these polyaddition reactions restricts their use for the synthesis of linear polyesters. These polyesters are mainly used for the production of composites, laminates and biodegradable polymers.4
Polyesters can also be prepared by ring-opening polymerization (ROP) using a variety of cyclic monomers and oligomers.13,14 This method can yield very high molecular weight polymers (> 105 g/mol) and is very fast.14 ROP also produces no by-products and allows for processing by a number of methods including pultrusion, resin transfer molding, and reaction injection molding.4
References & Notes
Harry R. Billica, Production of Polyethylene Terephthalate Using Antimony Trioxide as Poly-merization Catalyst, US Patent 2,647,885 (1953)
A. Ravve, Principles of Polymer Chemistry, 2nd Edition, Kluver Academic, New York 2000
Jan F. Kemkes, Process for the Preparation of Polyethylene Terepthalate, United States Patent 3,497,473 (1970)
A.A. Barot, T.M. Panchal, A. Patel, C.M. Patel, Arch. Appl. Sci. Res., 11(2): 19 (2019)
They are generally used at relative low concentrations. For example, typical levels of antimony trioxide are in the range of 0.01 - 0.02%2 and even lower amounts are used when titanium-based catalysts are employed.
Paal J. Flory, Kent, and Frederick S. Leutne, Method of Preparing Linear Polyesters, US Patent 2,589,688 (1952)
S.G. Sanadhya, S.L. Oswal and K.C. Parmar, J. Chem. Pharm. Res., 6(4), 705-714 (2014)
E.N. Zilberman A.E. Kulikova N.M. Teplyakov, J. Poly. Sci., 56 (164), 417-423 (1962)
Anthony J. East, Preparation of aromatic Polyesters by Direct Selfcondensation of Aromatic Hydroxy Acids, US Patent 4,393,191 (1993)
Wilfred Sweeny, Ester Interchange Polyester Preparation with Alkaly Metal Fluoride Catalyst, US Patent 4,782,131 (1988)
S. Cheng, B. Khan, F.K., M. Rabnawaz, Polymers, 10, 1113 (2018)
W.J. Blank, Z.A. He and M. Picci, J. Coat. Technol., Vol. 74, 33–41 (2002)
S. Watanabe, T. Miho, T. Fujii, Ring-opening polymerization of cyclic ε-caprolactone, US Patent 4,379,915 (1983)
J. Scheirs and T. E. Long, Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters, John Wiley & Sons, Chichester, West Sussex 2003
July 25, 2020