Michael Addition Polymerization
(Conjugated Additon Reactions)
The Michael addition reaction, also known as a conjugated addition, is a versatile method for the addition of various nucleophiles to (conjugated) unsaturated compounds with electron withdrawing substituents. This reaction was first discovered by Arthur Michael in 1887.1 It allows for the synthesis of a wide range of highly complex macromolecules under relative mild conditions and in a very efficient manner with often quantitative yield. Basically, any monomer with an activated double bond such as, α,β-unsaturated aldehydes/ketones, vinyl esters, vinyl sulfones, imidazoles, and maleimides etc. can undergo a Michael addition with a nucleophile such as thiol, amine or any stabilized carbanion:
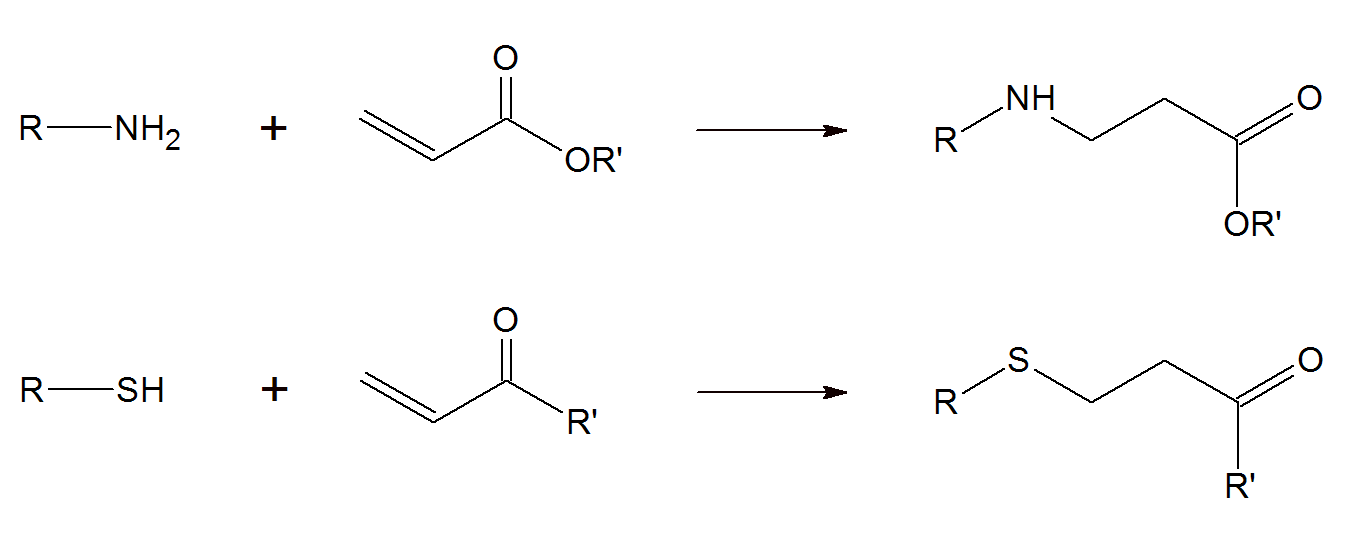
The reaction rate depends on the type of nucleophile (Michael donor) and activated double bond (Michael acceptor) and is highly affected by steric hindrance. For example, aromatic amines and amines with bulky side groups do not or only slowly react with α,β-unsaturated compounds such as simple vinyl ester and require a strong Lewis acid2, whereas many primary aliphatic amines and secondary amines without bulky side groups react readily with vinyl esters even without a catalyst. For example, diethyamine reacts faster than dipropylamine, which, in turn, reacts much faster than diisopropylamine.11 Aromatic amines such as benzenamine (aniline) are also very weak Michael donors because the phenyl ring is a strong electron withdrawing group, i.e. harsh reaction conditions are required.12 The same is true for most Oxa-Michael addition reactions such as addition of an alcohol to an ene compound which usually requires a strong catalyst and higher reaction temperatures. Typically, sulfur nucleophiles (also called mercaptans) (R-SH) react faster than secondary amines (R-NH2) followed by primary amines (R2NH) and alcohols (R-OH).10,11 However, the reaction order can change when bulky substituents are present. For example, cyclohexylamine is a stronger Michael donor than diisopropylamine.11 In the case of Michael acceptors, the reactivity decreases in the order11,12
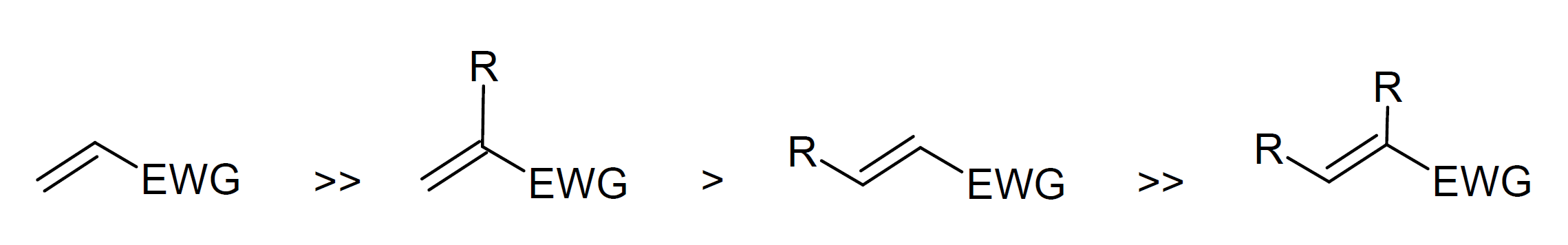
For example, most methacrylates have much lower reactivity than most acrylates due to the steric effect of the methyl group and are therefore less often used as Michael acceptors.
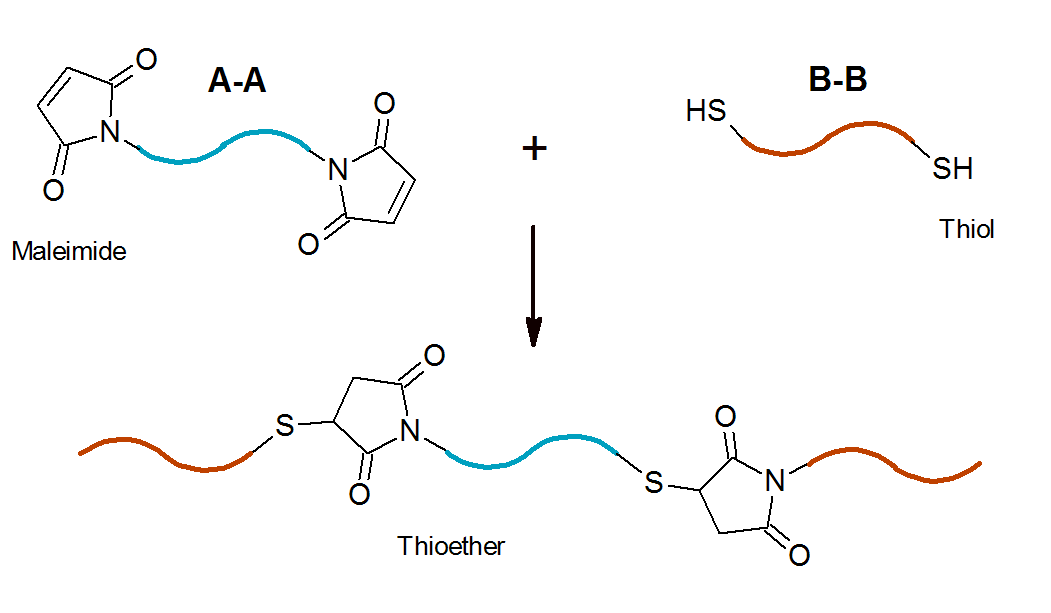
Michael addition reactions can be employed to prepare polymers of various architectures. The monomers of this type of
step-growth polymerization are molecules that contain conjugated bisdienes and bisdienophiles (A-A and B-B co-monomers).
A well known example is the addition of a bisthiol to a
bismaleimide:7
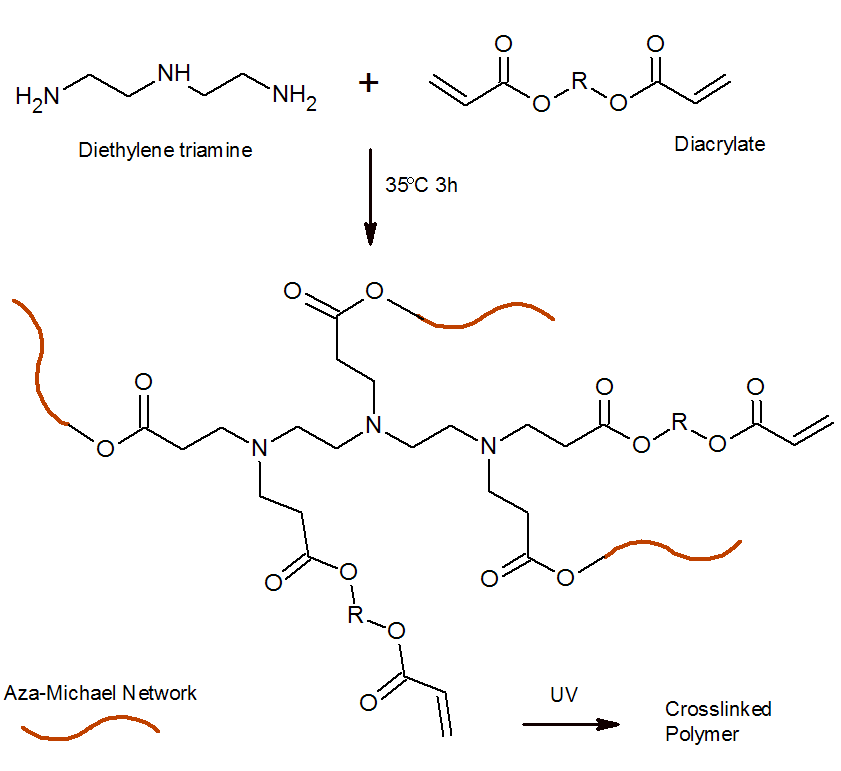
Michael addition reactions have also been applied to prepare thermosetting resins4. An example is the aza-Michael addition of a polyamine such as ethylenediamine, diethylenetriamine (DETA), or 1,3-diaminopropane to a diacrylate.5,6 These adducts undergo free radical photopolymerization when exposed to UV light. During the first stage, an off-stoichiometric amount of amino and acrylate groups are partially crosslinked through aza-Michael addition catalyzed by the tertiary amines formed during this reactions.8 The addition often proceeds at room temperature. In the second stage, the oligomers are fully crosslinked via free-radical photompolymerization of the excess amount of acrylic groups (dual cure).
References & Notes
A. Michael, Ueber die Addition von Natriumacetessig- und Natriummalonsaeureethern zu den Aethern ungesaettigter Saeuren, Journal fuer Praktische Chemie, Volume 35, Issue 1, 349-356, 21 March 1887
Feric chloride FeCl3, and aluminum chloride AlCl3 are excellent catalysts for the addition of primary and secondary amines to acrylates with near-quantitative yield.3
-
J. Cabral, et al., Tetrahedron Letters, Vol. 30, Issue 30, pp. 3969-3972 (1989)
-
G. Gonzales, et al., Polym. Chem., 6, 6987-6997 (2015)
-
Aza-Michael addition is the reaction of an amine with an electron poor C=C double bond.
-
G. González, X. Fernández-Francos, A. Serra, M. Sangermanoc and X. Ramis, Polym. Chem., 6, 6987 (2015)
-
Michael addition reactions are usually thermally reversible, which is known as a retro-Michael reaction. Thus, the adducts will revert to the starting compounds at elevated temperatures. In the case of a bismaleimide, the reversion takes place in the vicinity of 300°C. (US 20110152466)
-
Since amines act as both nucleophiles and base catalysts, aza-Michael addition reactions are highly efficient.
-
K.A. Shooshtarim. Thesis: Michael Addition of Amines to α-β Unsaturated Esters, University of Missouri-Rolla, 2000
-
Diehl, K., Kolesnichenko, I., Robotham, S. et al. Nature Chem 8, 968–973 (2016)
-
A. Genest, D. Portinha, E. Fleury, F. Ganachaud, Progr. Poly. Sci., Vol. 72, 61-110 (2017)
-
G.J. Noordzij, C.H.R.M. Wilsens, Front. Chem., Vol. 7, 729 (2019)
Revised April 26, 2020