Polymerization by Transition
Metal Complexes
A number of metal-containing compounds are capable of initiating radical vinyl polymerization. Some of the most effective initiators are transition metal chelates such as metal acetyl acetonates which have been intensively investigated in the 1960s and 1970s. The ability of metal chelates to produce free radicals was first observed by Arnett and Mendelsohn in 1962 when studying the oxidation of these compounds.1 They found that various metal beta-diketone chelates decompose at 100°C and produce rather stable radicals which initiate and promote polymerization of vinyl polymers. They postulated that heat induced initiation of metal acetyl acetonates is the result of an electron transfer from a ligand to the metal whereby the leaving acetylacetonate forms an inactive cyclic peroxide radical which in a subsequent step undergoes a ring open reaction:
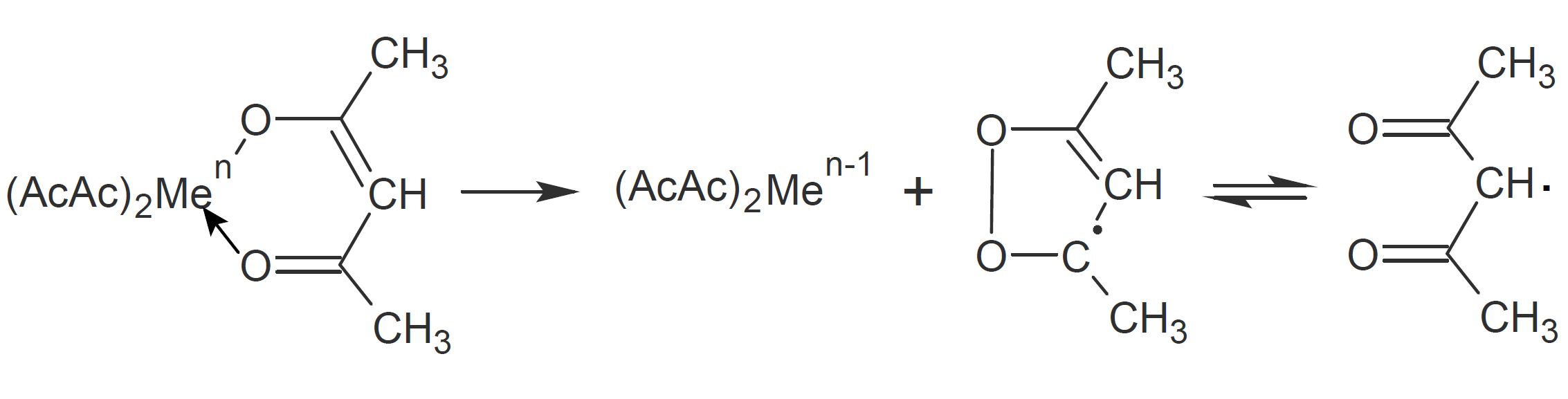
A highly selective and very effective initiator is manganic 1,1,1-trifluoroacetyl-acetonate [Mn(III)(facac)3],2 which can be employed in the polymerization of monomers that polymerize readily anionically. This includes monomers such as methyl methacrylate and acrylonitrile whereas monomers such as styrene and vinyl acetate are less susceptible to this type of polymerization. In fact, Mn(III)(facac)3 is a strong retarder of styrene polymerization.2 The proposed mechanism is heterolytic fission of a Mn-O bond followed by addition of the monomer to the anion. The resulting anion then reduces the manganese (III) to manganese (II) and forms a free-radical which is the initiator.
Vinyl polymerization can also be initiated with transition metal carboxylates such as cobalt(III) cyclohexanecarboxylate which is an effective initiator for the polymerization of methyl methacrylate.3 Its activity is higher than that of the corresponding salts of manganese (III) and copper (II). It was assumed that the radicals are generated by homolysis of the metal-oxygen bond in the carboxylate with participation of MMA monomer.
The formation of radicals by metal complexes can be greatly accelerated by a number of compounds that act as reducing agents. For example, some organic halides when combined with transition metal chelates are very effective for radical vinyl polymerization.4 Initiation proceeds by election transfer from metal to halide after the metal has been activated, for example by heat. In the case of metal chelates, halides are not necessary, but when present, often accelerated the process.5
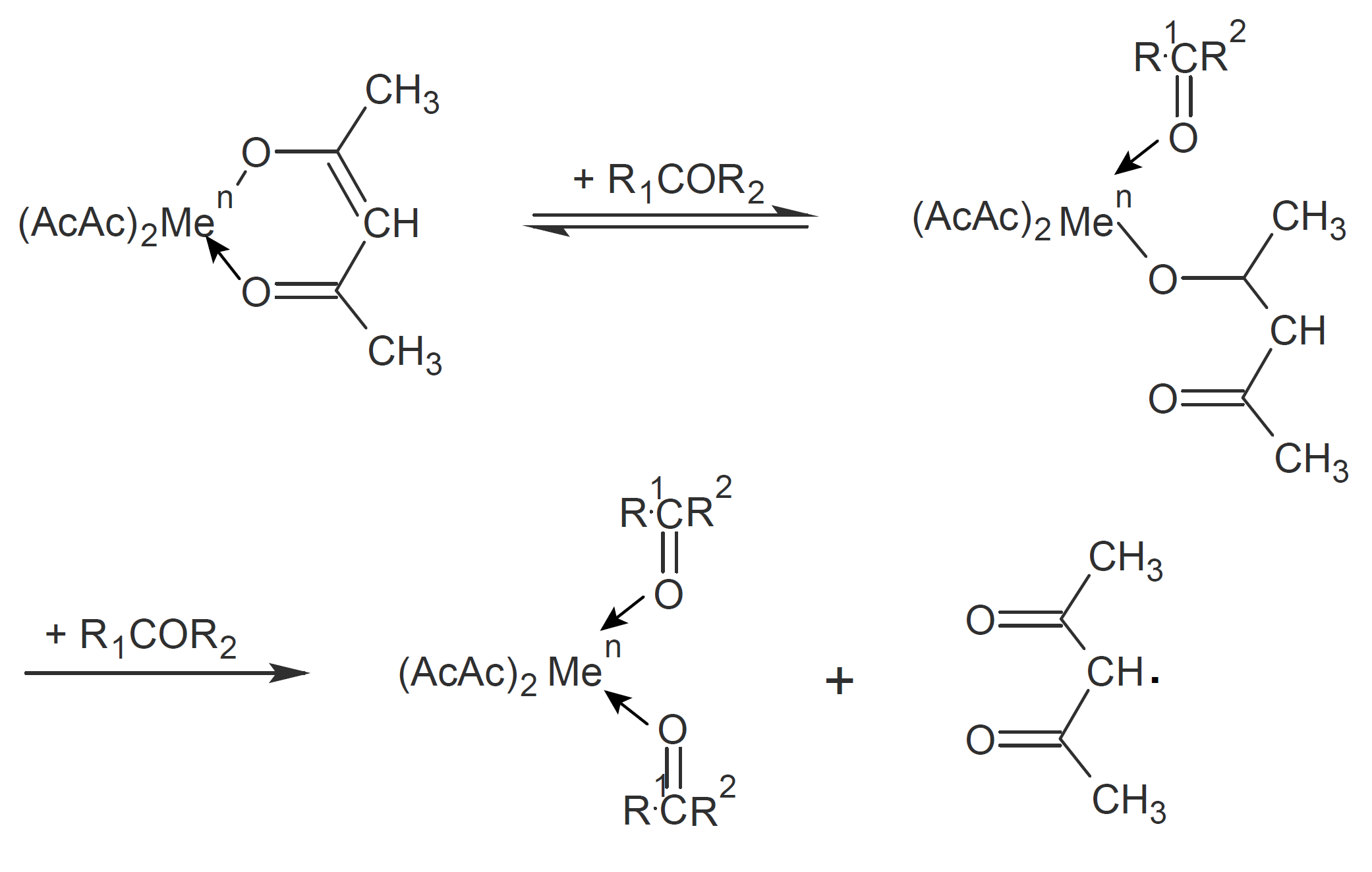
Kaeriyama5 found that some ketones and aldehydes have a similar accelerative effect on the polymerization of various vinyl compounds when combined with metal chelates such as manganese(III) acetylacetonate (Mn(AcAc)3); he observed that the conversion increases with an increase in concentration of these co-reactants and that the relation between the rate of polymerization and the square root of chelate concentration is linear without additive but concave in the presence of cyclo-hexanone.5 Kaeriyama also showed that the rate of reaction depends on the nucleophilicity of the carbonyl compound. The effectiveness of the metal chelate also depends on the substituents on the acetylacetone ligands and the type of solvent and monomer which suggests that the monomer and solvent participate in the initiation reaction.5 The initiation reaction is summarized schematically below; in the presence of a ketone, the activated metal chelate coordinates with ketones which weakens the bond between manganese and the acetylacetone ligand. Subsequent decomposition of the intermediate generates the radical which then initiates polymerization.
Another effective reducing agent for metal chelates are halogenated organic esters. For example, Endo and Yachi6 investigated the polymerization of MMA with Mn(AcAc)3 in the presence of methyl 2-bromopropionate. They suggested that this combination could proceed with a similar mechanism as transition metal mediated radical polymerization which would provide some control of the molecular weight of the growing polymers. To achieve a narrow molecular weight distribution, both a fast equilibrium between active and dormant growth centers and a fast initiation step are required. However, the latter condition is probably not met because a narrow molecular weight distribution was not achieved. Endo et al. noted that the structure of the halides plays an important role in controlling the molecular weight.
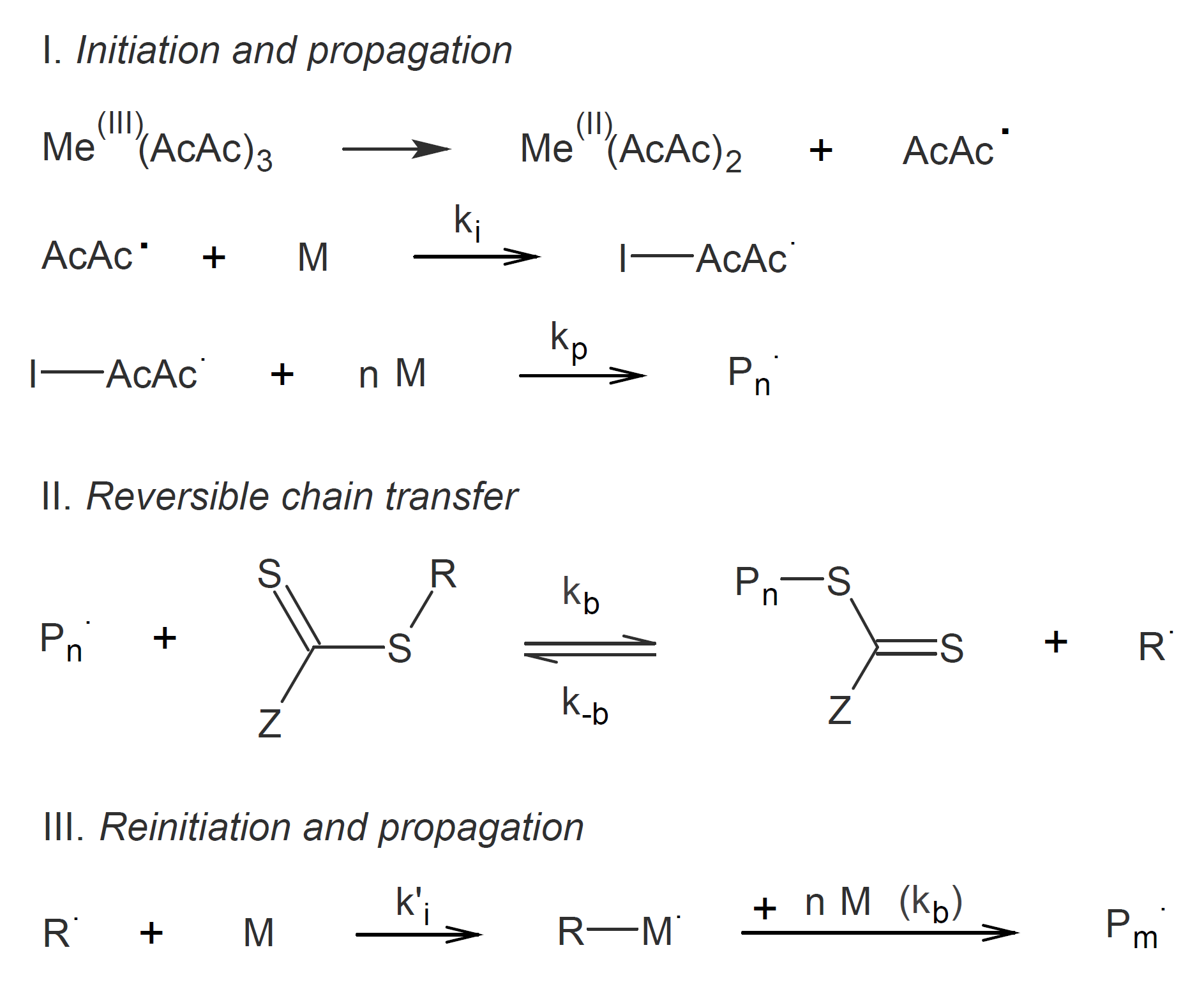
Mn(AcAc)3 has also been employed as an initiator in RAFT polymerization of vinyl acetate mediated by xanthate and methyl 2-(ethoxycarbonothioylthio) propanate and of methyl methacrylate at ambient conditions and styrene at 100°C using a typical RAFT agent such as dithiocarbamate and dithioesters.7 . Xu et al. showed that Mn(AcAc)3 is a versatile and superior RAFT initiator than azobisisobutyronitrile which could be used at low concentrations (1 %).8
Metal chelates have also been employed as organometallic catalysts in living atom transfer radical polymerization (ICAR ATRP). For example Wu et al.9 combined oil-soluble iron acetylacetonate (Fe(AcAc)3) with triphenylphosphine (PPh3) (ligand), ethyl-bromophenylacetate (initiator) and ACHN (thermal initiator) to polymerize methyl methacrylate. The living feature of this polymerization system was confirmed by subsequent chain extension. This polymerization system has the important advantage over conventional ATRP that only small amounts of a relatively inexpensive and low toxic catalyst are needed.10
References & Notes
E.M. Arnett and M.A. Mendelsohn, J. Am. Chem. Soc. 84, 20, 3821–3824 (1962)
C.H. Bamford and D.J. Lind, Proc. Roy. Soc. A. 302, 145-165 (1968)
S. Aoki, T. Otsu et al., Bull. Chem. Soc. Jpn., 42(9) 2574-2577 (1969)
C.H. Bamford and K. Hargreaves, Trans. Faraday Soc. 63, 392 (1967)
K. Kaeriyama, Bull. Chem. Soc. Jpn., 43, 1511-1516 (1970)
K. Endo and A. Yachi, Polym. J., Vol. 34, No. 5, pp 320—324 (2002)
Z. Zheng, W. Wang, Y. Zhou, Z. Zhang, X. Zhu, Polym. Chem. 5(1), 37-42 (2014)
Y. Xu, J. Sun, H. Chen, L. Bai, Q. Tao, L. Yu, Y. Wang, Polym. Chem. 53(11), 1305-1309 (2015)
J. Wu, X. Jiang, L. Zhang, Z. Cheng, X. Zhu, Polymers 8(2), 29 (2016)
The amount of catalyst can be significantly reduced because the continuously produced free-radicals reduce the inactive transition metal complexes in higher oxidation state.
February 19, 2021