Depolymerization
Polymers can undergo significant chemical changes over time when exposed to high temperatures. These changes have a dramatic effect on the service life and properties of the polymeric material. The deterioration of the physical and mechanical properties is often the result of bond breaking in the polymer backbone (chain scission) which may occur at the chain ends or at random positions in the chain. In the case of chain-end scission, monomers are released. This process is known as unzipping, depropagation or end-chain depolymerization. Random main chain scission, on the other hand, leads to the formation of both monomers and oligomers (short chains with ten or fewer monomers). This process can be considered the reverse of a step-growth polymerization. Both reactions compete with cross-linking reactions, chain stripping of side groups as well as with substituent and cyclization reactions. Which of these mechanisms dominates depends on the type of polymer and temperature.
Polymers with no or only a single (small) substituent in the repeat unit usually decompose by random-chain scission rather than end-chain scission. This is the case for polyethylene, polypropylene and polymethyl acrylate. On the other hand, end-chain scission is usually the predominant decomposition mechanism in polymers with two substituents at the same carbon atom because the (large) side groups interfere with hydrogen abstraction which is known as steric hindrance. Thus disubstituted polymers like poly(methyl methacrylate), poly(α-methylstyrene), and poly(methacrylonitrile) usually undergo end-chain scission with high monomer yield (≥ 90%) whereas polymers with a single large substituent are susceptible to both random chain scission and end-chain scission. However, the monomer yield usually does not exceed 50 percent.
Depolymerization involves a combination of some or all of the following reaction steps:
Initiation
Depolymerization starts with the
homolytic fragmentation of atoms in the chain backbone by either random-chain scission or end-chain scission
which produces free radicals.
Pn → Rr· + Rn-r· (Random
Scission)
Pn → Rn-1· + R1· (End-chain
Scission)
where Pn is a polymer molecule of degree of polyemrization n and Rr· is a polymer radical of length s.
Propagation
The propagation reactions of depolymerization are often called depropagation reactions. There are three different types:
Rn· → Rn-r· +
Rr (Intramolecular
H Transfer)
Rn· → Rn-1· + M (unzipping)
Rs·
+ Pn → Ps + Rn-r + Pr (Intermolecular
H Transfer)
Unzipping produces monomers whereas inter- and intra-molecular hydrogen transfer usually results in fragments of larger size.
Termination
The three most common termination reactions are
Rn· → Pn (Unimolecular
termination)
Rr· + Rs· → Pr+s (Recombination)
Rr· + Rs· → Pr + Ps (Disproportionation)
A first order termination reaction is theoretically not possible because it is impossible to remove a radical without adding or removing a hydrogen atom as well. However, a termination reaction can be quasi-first order if the other compound that recombines with the chain radical is so abundant that the termination reaction appears not to be affected by its concentration.
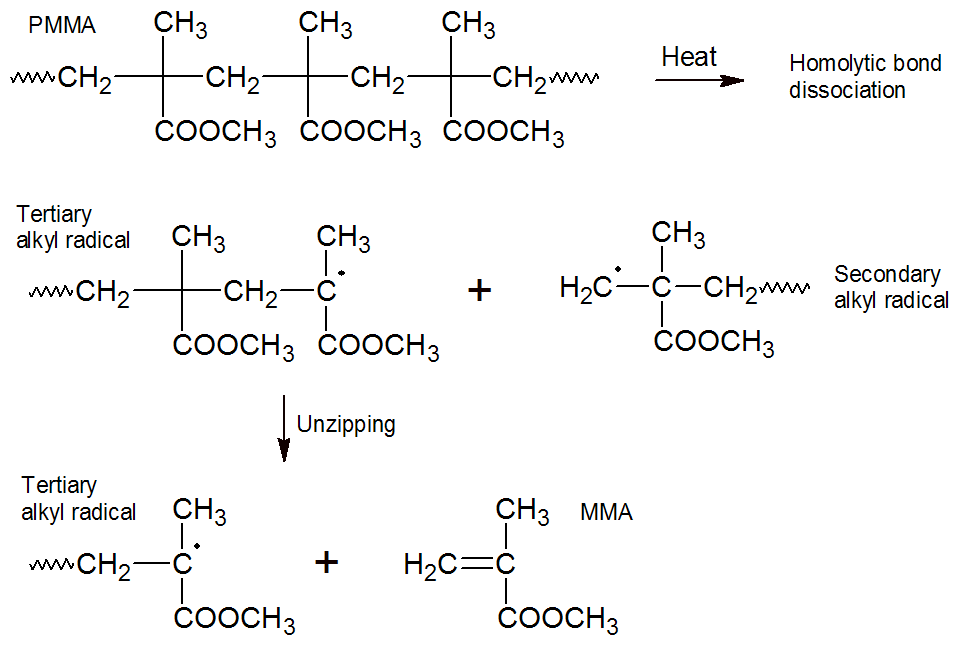
Polymethyl methacrylate (PMMA): A well known example of end-chain depolymerization (unzipping) with high monomer yield is the decomposition of polymethyl methacrylate (PMMA). This polymer starts to decompose at about 350°C (660°F).2 Random-chain fragmentation is the main intitiation step in the early stages and in the later stages end-chain scission which is usually of first order. The main propagation step is unzipping to monomer which releases large amounts of methyl methacrylate (> 90%). At even higher temperatures and conversion rates decoposition proceeds by a complex series of reactions which lead to the formation of other decomposition products such as butene, methacrylic acid anhydrides.
Styrenic Polymers: Polystyrene does not undergo
any appreciable weight loss below 280°C though there is a reduction
in molecular weight due to chain scission. The thermal decomposition
process of polystyrene is rather complex including end chain scission,
carbon-hydrogen bond cleavage, and free radical recombination. The main volatile products are
monomer (40 - 45%) with decreasing amounts of
dimer, trimer, tetramer, and pentamer. The formation of monomer is mainly caused by a free radical initiated end-chain scission whereas styrene dimers, trimers, and tetramers are generated by intramolecular transfer reactions.
Poly(α-styrene) is less thermally stable than polystyrene. The addition of the
α-methyl group provides enough steric hindrance that only monomers
are
generated by end-chain scission (unzipping)
with a yield of about 95%3
Linear and Branched Polyolefins: Thermal decomposition of linear polyethylenes usually starts between 215 and 230°C whereas branched PE decomposes at even lower temperatures. Usually the greater the branching is the greater the rate of weight loss.Thus thermal stability decreases in the order
CH2CH2 > CH2CHCH3 > CH2CHR > CH2CR2
The main decomposition mechanism of polyethylene is
random chain scission. Major volatile decomposition products are low
molecular weight alkanes such as propane > ethane > butane etc. which are produced
by recombination reactions, and low molecular weight alkenes such as propylene >
ethylene = Hexene-1 > butene-1 which are produced by chain-end
unzipping and molecular radical transfer followed by decomposition
reactions.
In polypropylene (PP) every second atom of the polymer backbone is a
tertiary carbon atom. Since these are more prone to attack than polyethylene,
PP is less thermally stable than PE. However, random
chain scission and transfer does not produce much volatile compounds
below 450°C.4
Notes & References
The same is true for polymers where all hydrigen atoms have been replaced with atoms that form strong bonds with the backbone carbon atoms. A well known example is polytetrafluoroethylene (PTFE) which decomposes mainly via end-chain scission (unzipping).
C.L. Beyler and M.M. Hirschler, Thermal Decomposition of Polymers in SFPE Handbook of Fire Protection Engineering 2, Chapter 7, 110-131 (2002).
-
C.H. Bamford, C.F.H. Tipper, Comprehensive Chemical Kinetiks, Vol. 14, Degradation of Polymers, Elsevier, New York, 1975
A. Brzozowsja-Stanuch, S. Rabiej, J. Fabia, J Nowak, Polimery, 59, p. 302-307 (2014)