Carbonyl Addition Reactions
Carbonyl groups can be found in a myriad of natural occurring and synthetic organic compounds either as aldehydes and ketones or as part of other functional groups including amides, carbonates, carboxylic anhydrides and carboxylic esters. Due to their polar nature, carbonyl groups are susceptible to nucleophilic and electrophilic attack, but unlike carbon double bonds, C=O bonds are not very susceptible to polymerization by radical initiators due to the instability of the carbonyl radical. However some carbonyl containing compounds, in particular aldehydes, readily undergo ionic chain growth polymerization.1 Some ketones and aldehydes also readily react with other functional groups and can be polymerized by step-growth if at least one of these compounds is difunctional. Below, some common nucleophilic and electrophilic carbonyl addition reactions that can be employed to synthesized step-growth polymers are briefly discussed.
Reactions with Alcohols
Polyacetals are probably the most important type of carbonyl derived polymers which are frequently produced by anionic polymerization of aldehydes. An alternative route to polyacetals is the reaction of aldehydes (or ketones) with diols in the presence of an acid catalyst.2
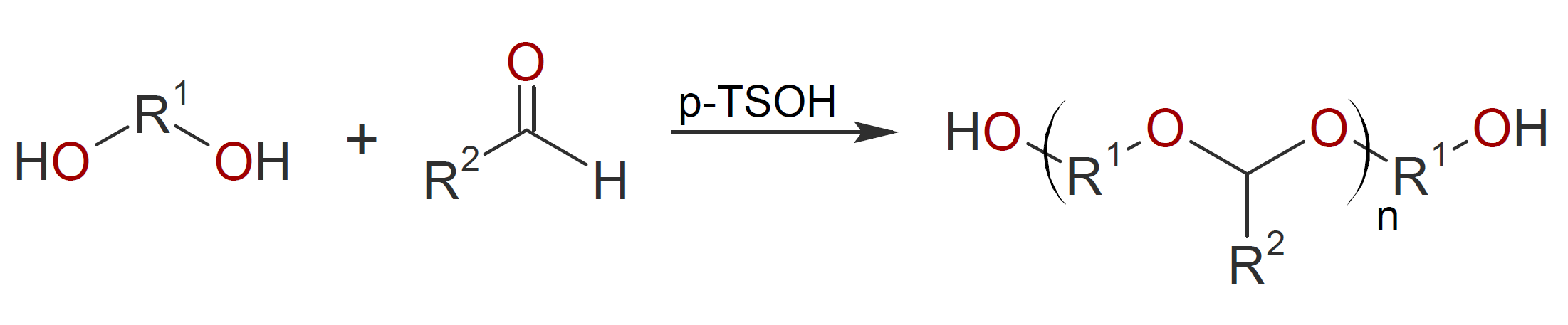
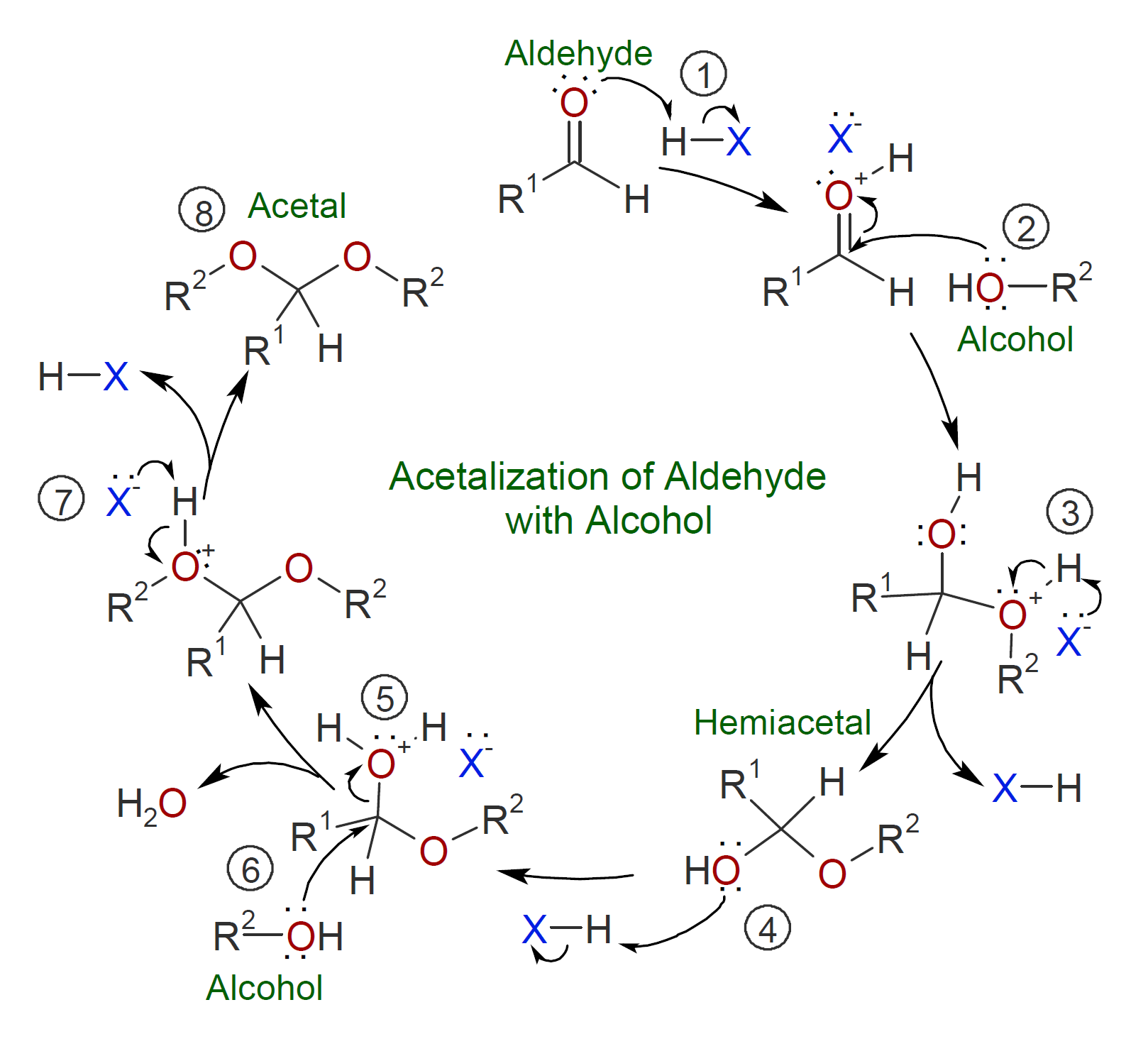
This type of polymerization proceeds by repeated acetalization (ketalization) where the carbonyl is activated by a Bronsted acid catalyst which produces an oxonium ion that promotes the nucleophilic addition of an alcohol to form a hemiacetal.3 Due to the instability of the hemiacetal under acidic conditions, a second alcohol is added with water as a by-product which needs to be removed during the reaction since acetal formation is reversible. The most common catalyst is p-toluene sulfonic acid (PTSA) which is considered the gold standard to catalyze the formation of polyacetals.2 The general mechanism for acetalization of aldehyde with alcohol using acid as a catalyst is shown below.2
With the exception of polyoxymethylene (polyformaldehyde), polymers produced by this route have found very little commercial use probably due to the low thermal stability. However, in the future polymers produced
via acetalization might find more applications if the need for renewable sourcing and biodegradable plastics increases. Novel polyacetals are also explored as drug delivery systems and
for various (bio)medical applications due to the reversibility of acetal formation.4,5
Reactions with Amines and Derivatives
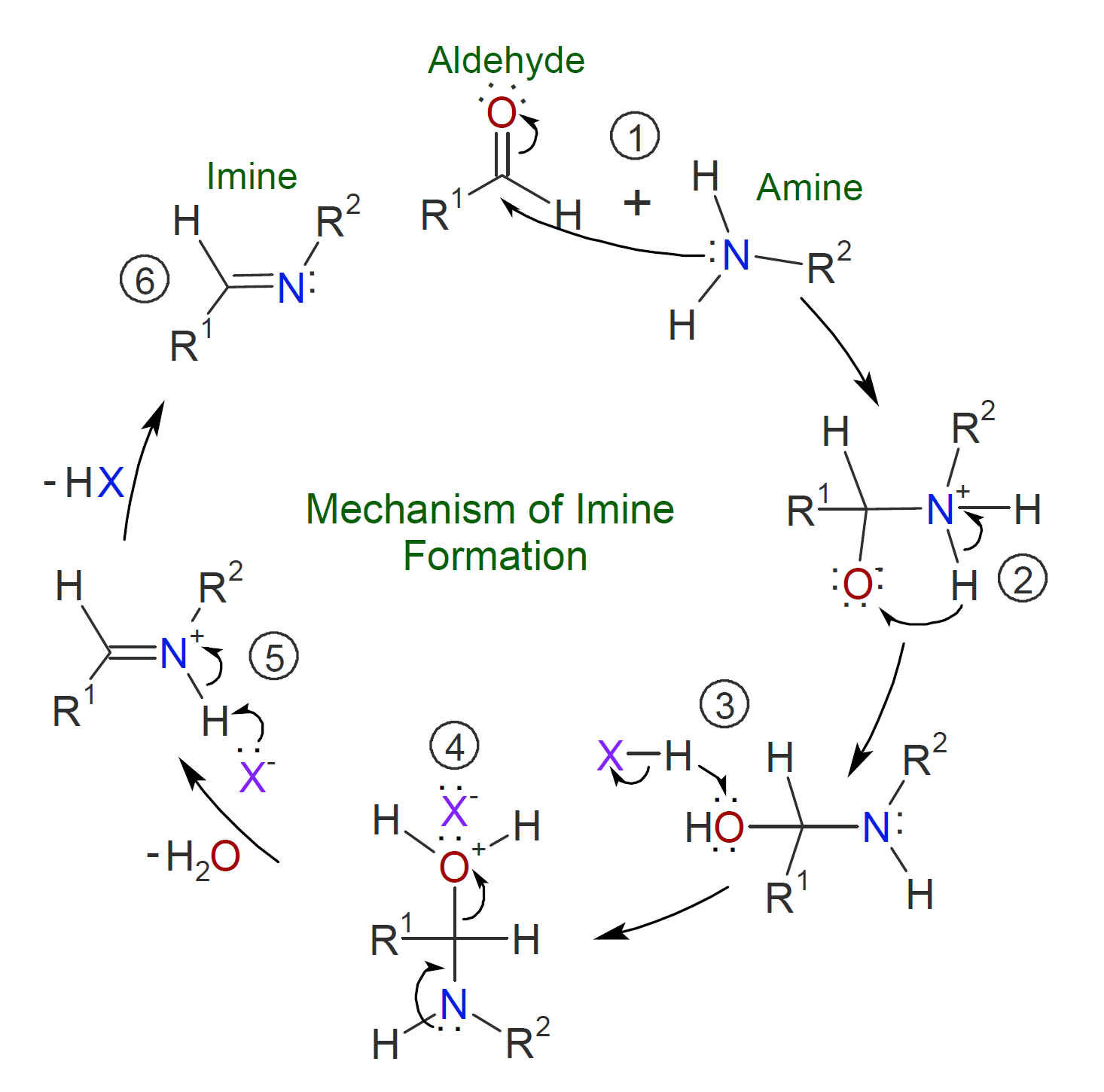
The carbonyl group of aldehydes and ketones readily reacts with primary and secondary amine which leads to essentially quantitative formation of imines and enamines, respectively.6-8 The general mechanism of imine formation by reaction of an aldehyde with an alcohol is shown below. The (reversible) imine formation starts with nucleophilic addition of the primary amine to the carbonyl group of the aldehyde (or ketone). In a second step, a proton is transferred from the nitrogen to the oxygen atom resulting in a neutral amino alcohol called a carbinolamine. Subsequent acid protonation of the carbinolamine oxygen converts the OH group into a better leaving group which is then eliminated as water producing an iminium ion. Deprotonation of the nitrogen yields the final imine product.
A wide variety of other compounds with -NH and/or -NH2 moieties react with aldehydes and ketones when an acid catalyst is present (see table below). The reaction mechanism of those containing primary amine moieties is typically very similar to the one described above yielding C=N- bonds and water as a by-product. The reaction with secondary amines, however, proceeds differently. Due to the lack of a second hydrogen on the nitrogen, the formation of an imine is not possible. Instead, enamines (alkene + amine) are formed after nucleophilic addition of the amine. Like acetal formation, this reaction is an acid-catalyzed reversible reaction:
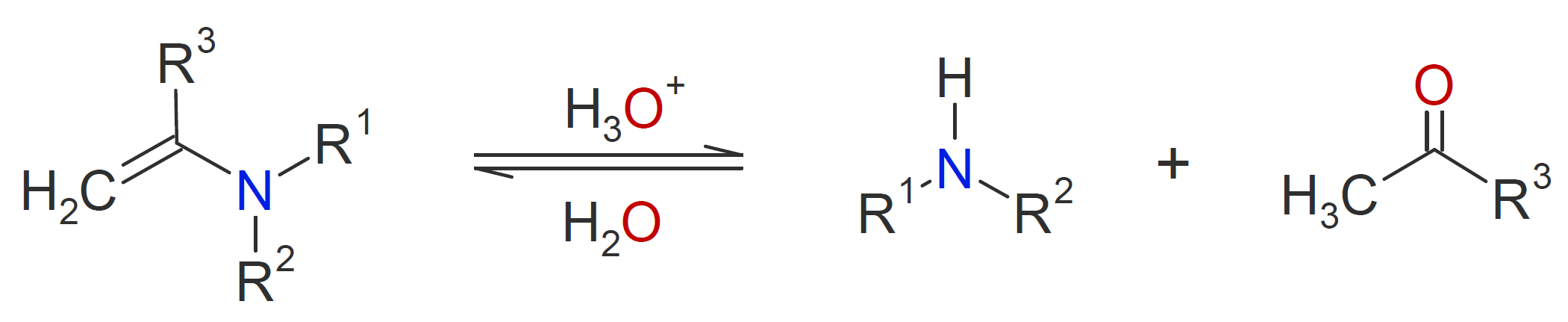
Thus, enamines are easily converted back to their carbonyl and amine precursors by acid-catalyzed hydrolysis.
| Reactions of Carbonyl Compounds with Amine and its Derivatives |
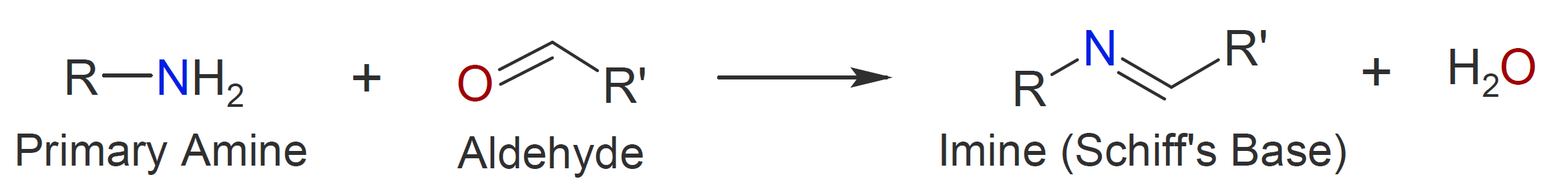 |
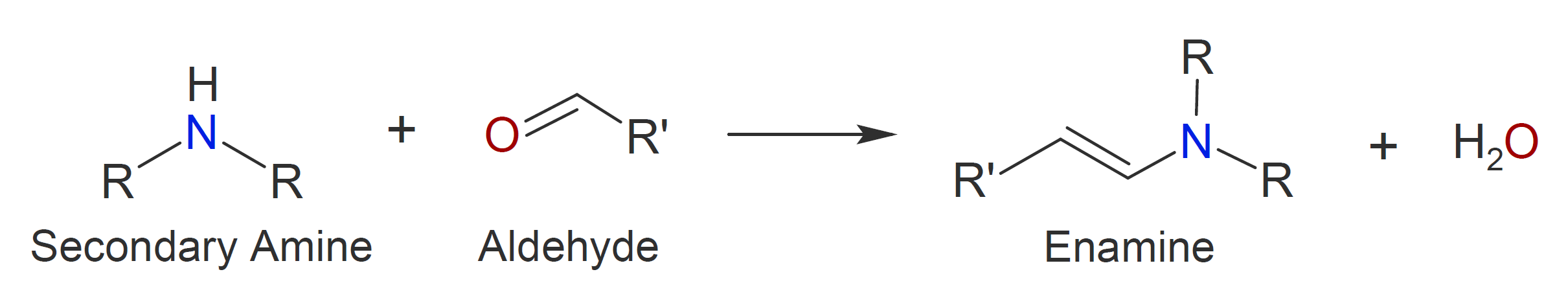 |
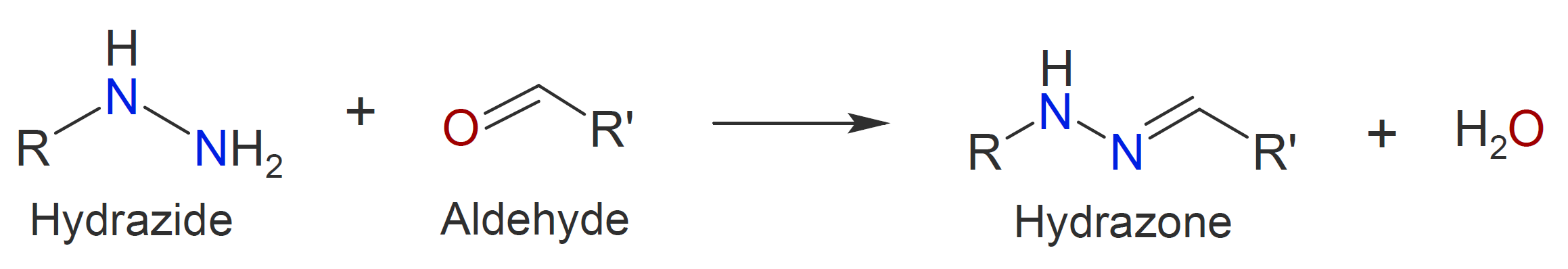 |
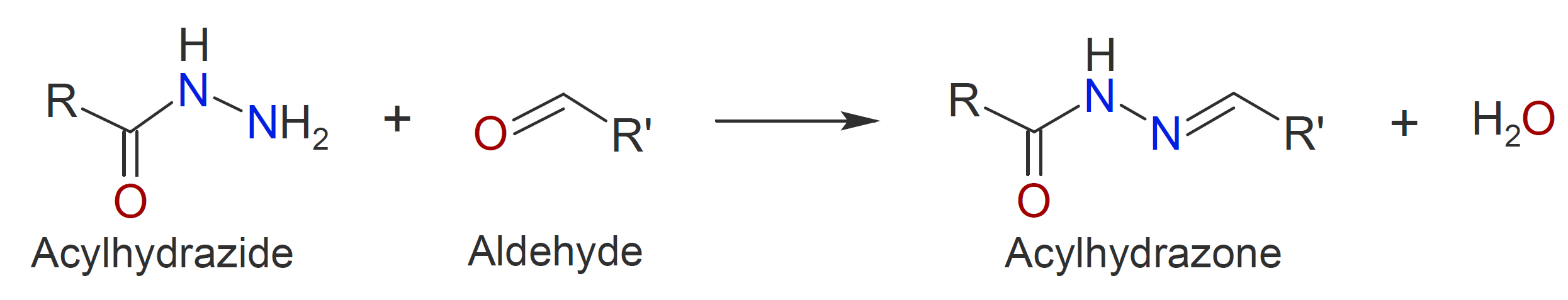 |
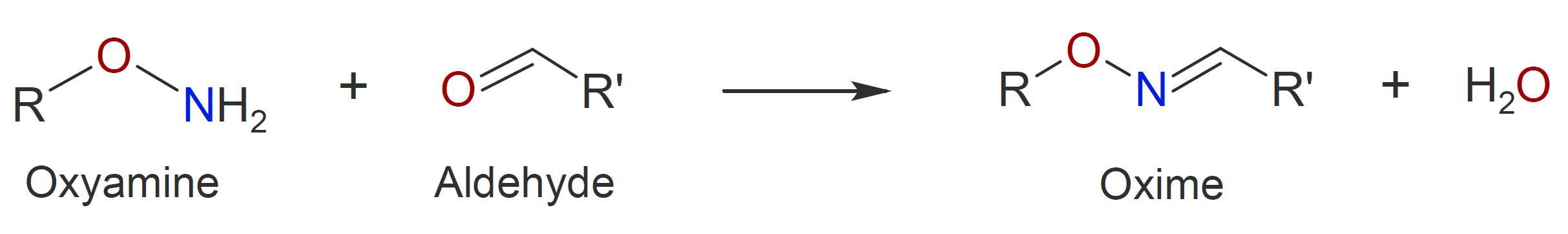 |
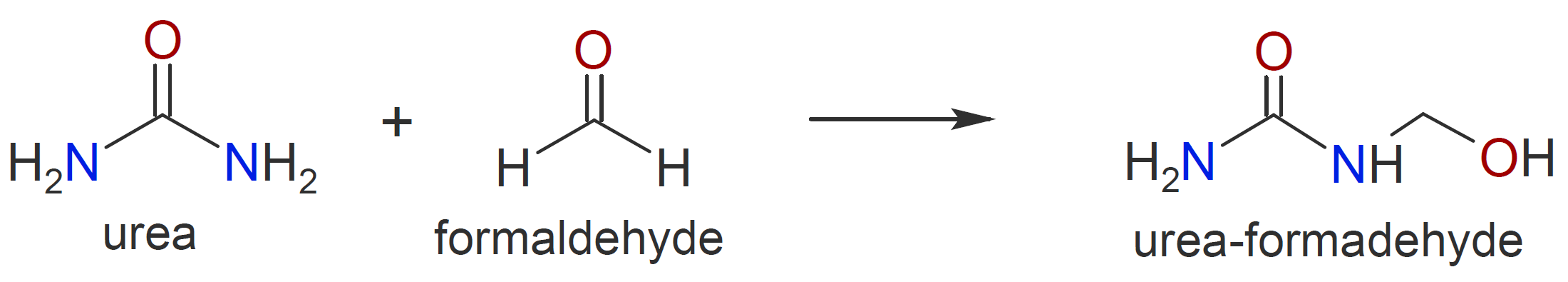 |
Note, the reaction of formaldehyde with urea will be discussed elsewhere.
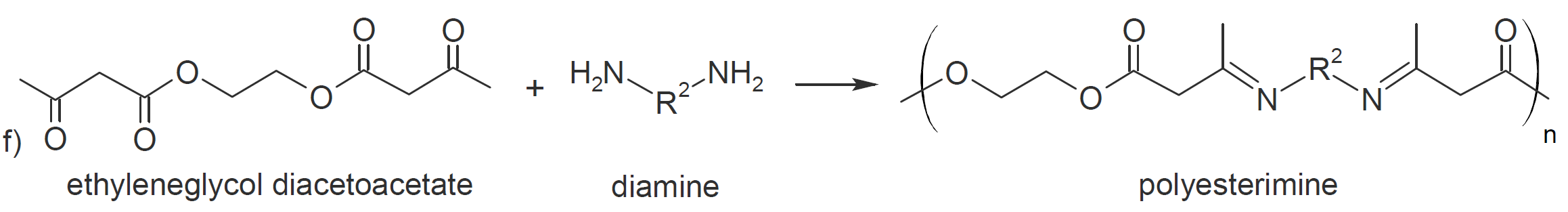
A wide variety of polymers with a broad range of properties and (potential) applications can be synthesized via carbonyl addition step-growth polymerization. Some examples are shown in the table below.
Linear polyacetals can be synthesized via direct polyacetalization (polyketalization), that is, by reacting equimolar amounts of an aldehyde or ketone with a diol in the presence of an acid catalyst (typically
a Bronsted acid). To drive the reaction to completion, the condensate (water) needs to be removed from the reaction mixture which can be achieved by (azeotropic) distillation. A major limitation of this type
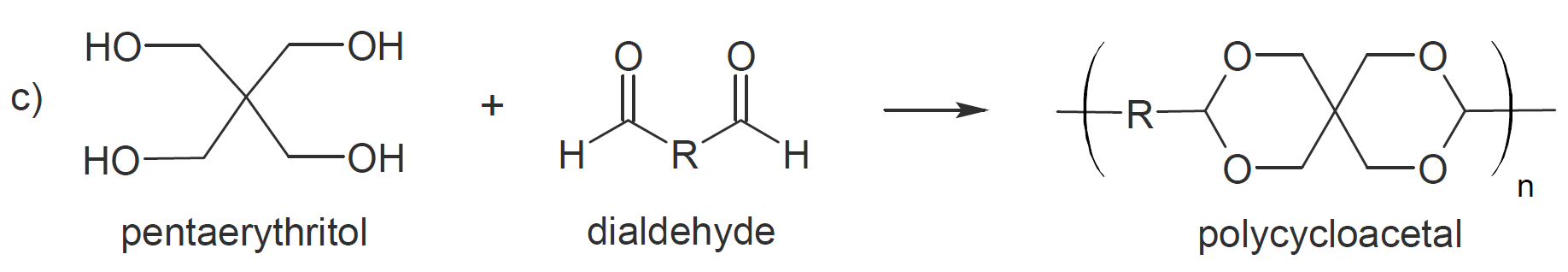
of polyacetalization is the formation of cyclic acetals as a site product which greatly lowers the average molecular weight.3 However, when reacting tetraols with dialdehydes or diketones this tendency
can be used to great advantage to produce polycycloacetals (polymer b and c).9-11
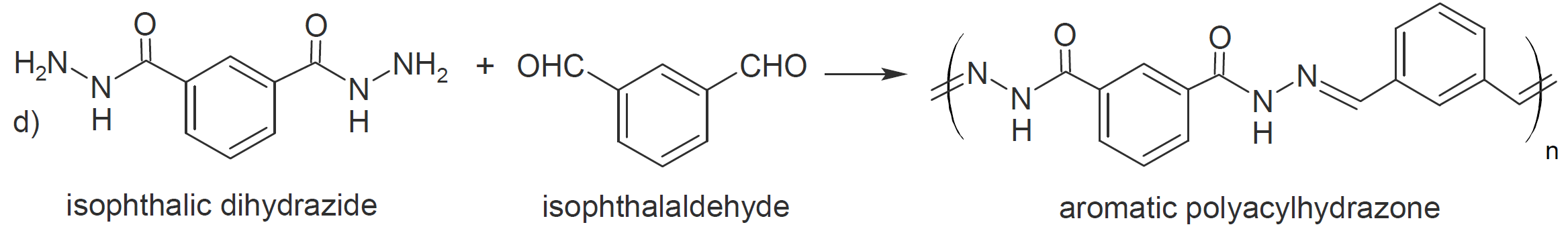
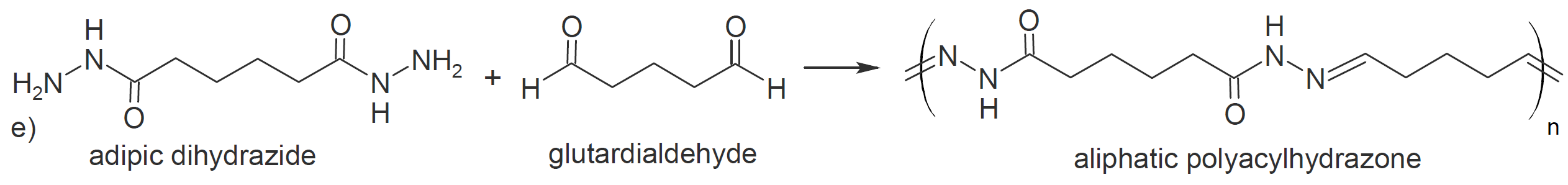
Another class of carbonyl addition polymers can be produced by polycondensation of dihydrazides with dialdehydes (or diketones) under mild conditions, yielding polyacylhydrazones in high yield (d and e).12 This class
of polymers possesses many attractive properties due to both the reversibility of the imino bonds and the hydrogen-bonding sites of the amide groups, resulting in polymers with properties similar to polyamides but
that are capable of exchanging their components.13 They are often called dynamers since they are dynamic by nature (supramolecular) and by design (molecular) due to the presence of covalent reversibility in the polymer
backbone.12
| Step Growth Polymers Derived from Carbonyl Addition Reactions |
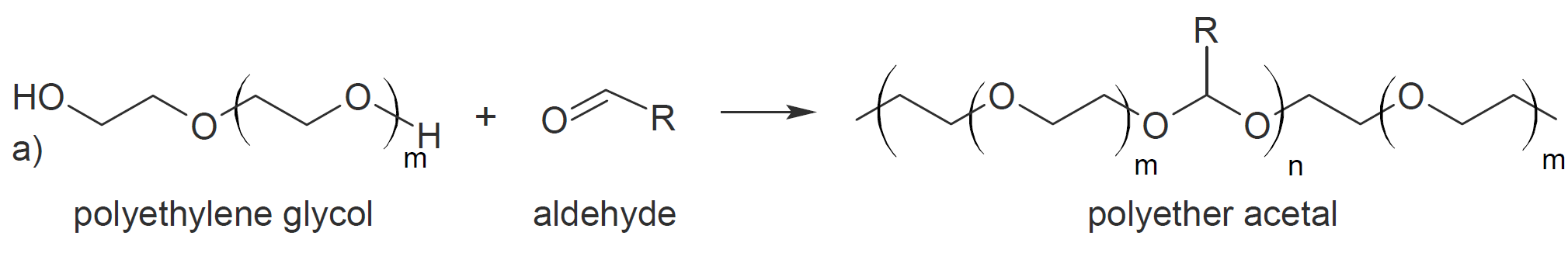 |
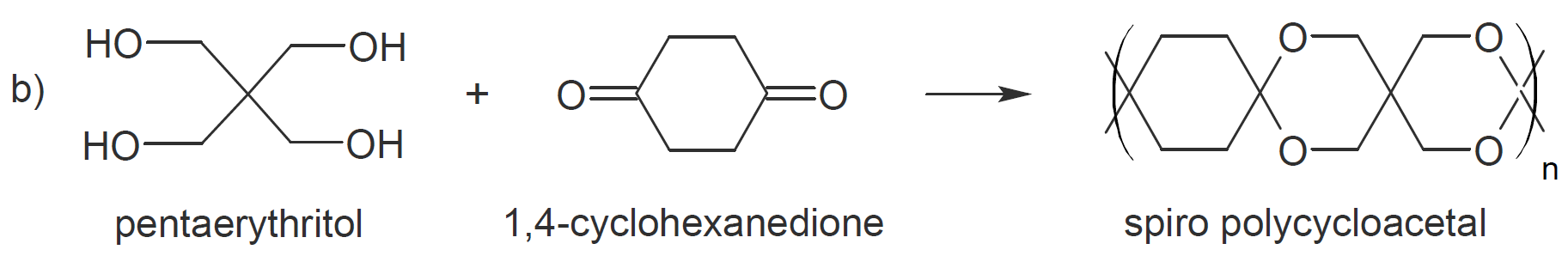 |
 |
 |
 |
 |
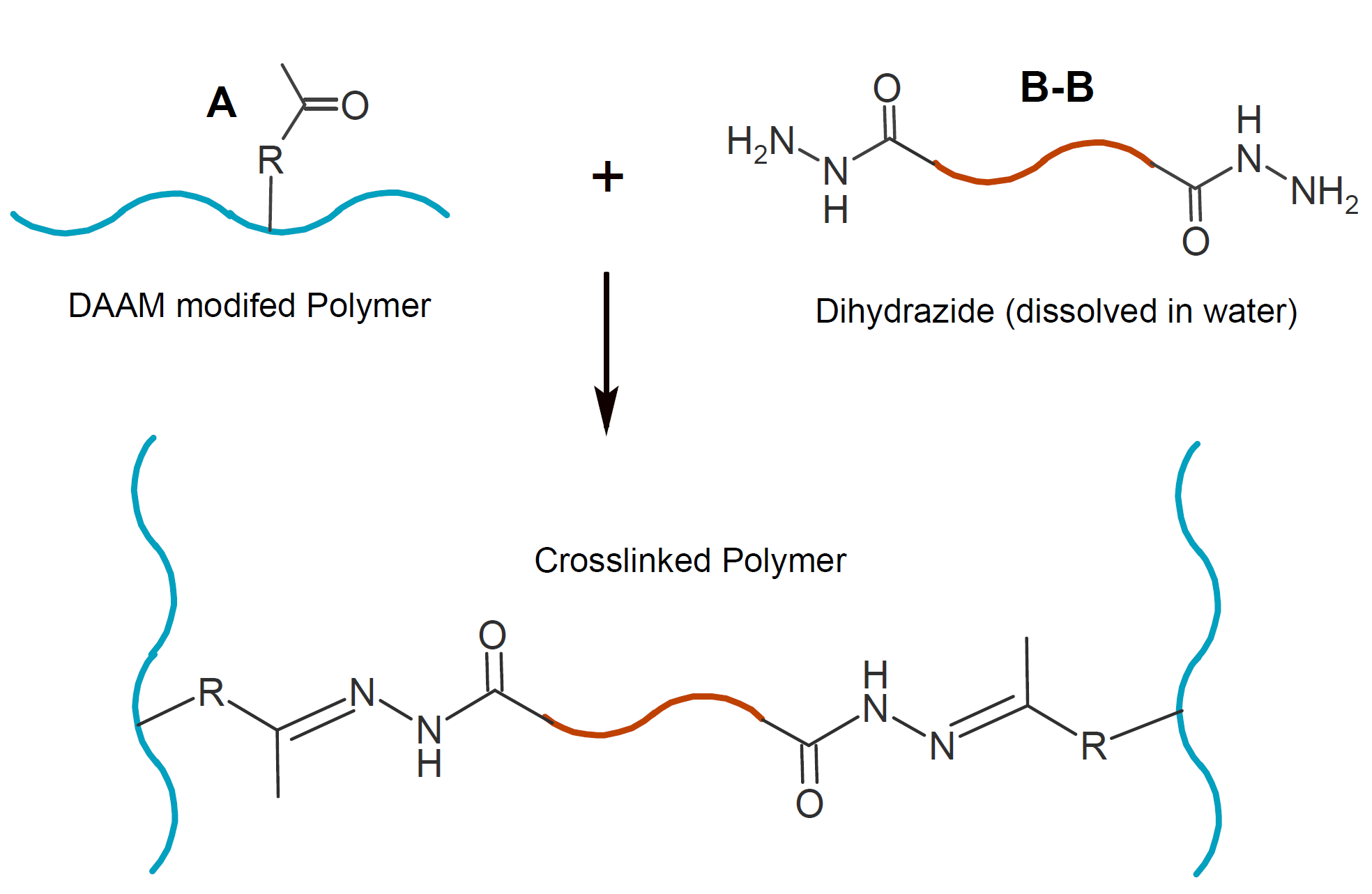
Condensation of hydrazides with ketones is also employed to crosslink water-based polymer latex.14 Polymers with pendant reactive ketone groups can be prepared either by copolymerization with vinyl monomers or by acetoacetylation of polymers / oligomers after synthesis. A common ketone used for this application is diacetone acrylamide (DAAM). Crosslinking occurs by reaction of pendant ketone moieties on the (acrylic) polymers with water soluble dihydrazides under neutral and acidic conditions initiated by evaporation of water and ammonia (NH3) during film-formation. This technology provides crosslinkable polymer latex with excellent long-term stability.
References, Notes & Further Readings
- O. Vogl, J. Poly. Sci.: Part A: Poly. Chem., Vol. 38, 2293-2299 (2000)
- A. Bossion, K.V. Heifferon, L. Meabe, N. Zivic, D. Taton, J.L. Hedrick, T.E. Long, H. Sardon, Prog. Polym. Sci., Vol. 90, pp 164-210 (2019)
- In general, aldehydes are more reactive toward nucleophilic attack than ketones.4
- A. Hufendiek, S. Lingier and F.E. Du Prez, Polym. Chem., 10, 9-33 (2019)
- M.J. Heffernan and N. Murthy, Bioconjugate Chem., 16, 6, 1340-1342 (2005)
- David R. Klein, Organic Chemistry, John Wiley and Sons, 2017
- Seyhan N. Ege, Organic Chemistry, Houghton Mifflin Comp., New York 1999
- Marc Louden & Jim Parise, Organic Chemistry, Freeman & Company, New York 2021
- S.M. Cohen and E. Lavin, J. Appl. Polym. Sci. 6(23), 503-507 (1962)
- S.M. Cohen, C.F. Hunt, R.E. Kass and A.H. Markhart, J. Appl. Polym. Sci. 6(23), 508 (1962)
- W.J. Bailey and A.A. Volpe, J. Polym. Sci., Part A-1: Polym. Chem. 8(8), 2109-2122 (1970)
- W.G. Skene and J.M.P. Lehn, PNAS, 101 (22), 8270-8275 (2004)
A dynamic polyacylhydrazone analog to Nylon 6,6 can be synthesized by polycondensation of adipic dihydrazide with hexan-1,6-dial (OHCO(CH2)4OCHO) which could be termed dynalon.12
- N. Kessel, D.R. Illsley, J.L. Keddie, J Coat Technol. Res. 5, 285-297 (2008)