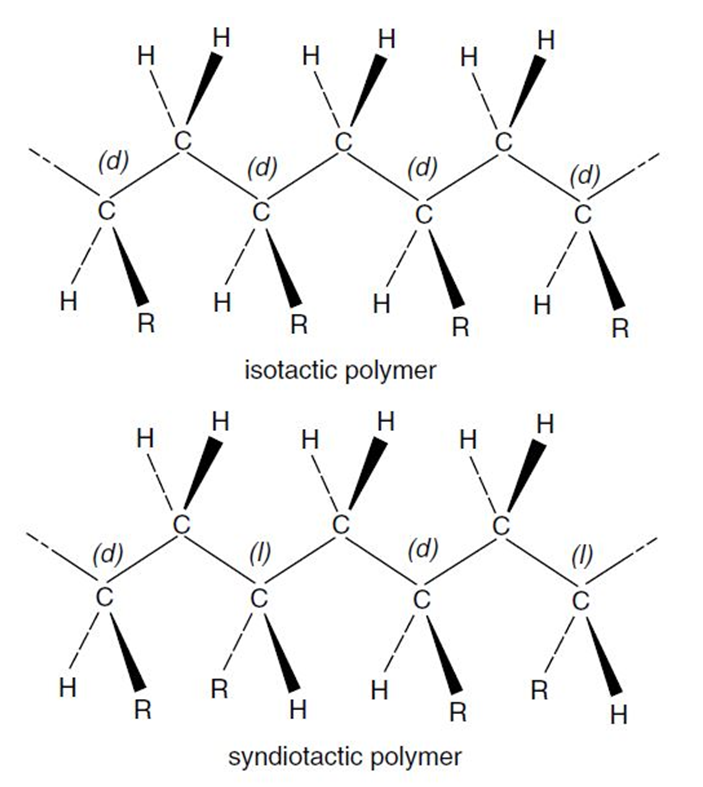
Steric Arrangement in Polymers (Tacticity)
The physical properties of a polymer depend not only on the type of monomer(s) that make up the polymer but also on the stereochemical arrangements of the atoms. In a linear asymmetric polymer chain, the pendant groups can either be arranged into orderly configuarations or they can be completely random. The steric order is called tacticity. If all chiral centers have the same configuration, the arrangement of the side groups is called isotactic, and if every other chiral center has the same arrangement, it is called syndiotactic, whereas a random arrangement of the side groups is called atactic or heterotactic.
Stereospecific macromolecules can also be polymerized from 1,2-disubstituted monomers. For example disubstituted olefins with two different side groups have two asymmetric carbon atoms in each repeat unit. The stereoisomers of these repeat units are called diastereoisomers (diastereomers). They are non-mirror image and non-superimposable stereoisomers whereas enantiomers with one asymmetric carbon have non-superposable mirror images.
The pysical properties such as melting range, glass transition temperature, solubility, etc. will depend on the stereospecific arrangement of the side chain substituents in the polymer chain. For example, the difference of the glass transition temperature (Tg) of syndiotatic and isotatic poly(methyl methacrylate) lies in the range of 115 K. This has been confirmed by a theoretical derivation based on the Gibbs–Di Marzio (1958) theory of the glass transition1. For polymers with only one substituent group other than hydrogen, the effect of tacticity on the glass transition temperature is much less pronounced. For example, for polystyrene and poly(alkyl acrylates), the variations in Tg are only around 20 K, whereas for poly(α-chloro acrylates) and for poly(α-methyl styrene) variations of 90 K and 65 K, respectively, have been observed. The explanation for this behavior lies in the added steric repulsion to rotation due to the presence of the asymmetric double-sided groups on alternate chain backbone atoms, which increases the stiffness of the polymer significantly compared to an atactic polymer. For example, the extended planar zigzag configurations and the different helical forms are not obeserved in highly syndiotactic chains. Also, the greater order in syndio and isotactic chains favors crystallization, that is, tactic polymers are often (partially) crystalline.3,4 The table below gives glass temperatures for some syndiotactic, isotactic and atactic (meth)acrylate polymers.
| Polymer | Tg(atactic) | Tg(isotactic) | Tg(syndiotactic) |
| Poly(methyl acrylate) | 281 | 272 (283) | 299 |
| Poly(ethyl acrylate) | 249 | 253 (248) | 263 |
| Poly(methyl methacrylate) | 378 | 319 (317) | 433 (432) |
| Poly(n-butyl methacrylate) | 293 | 249 (250) | 361 (361) |
| Poly(isopropyl acrylate) | 267 | 264 (262) | 285 (278) |
| Poly(methyl α-chloroacrylate) | 416 | 353 (358) | 452 (450) |
| Poly(isopropyl α-chloroacrylate) | 363 | 321 (341) | 392 (409) |
References
- E. A. DiMarzio and J. H. Gibbs, J. Chem. Phys. 28, 807 (1958); 28, 373 (1958)
- J. Biros, T. Larina, J. Trekoval, J. Pouchly, Coll. Poly. Sci., vol. 260, pp 27-30 (1982)
- F.E. Karasz, H.E. Bair, and J.M. O'Reilly, J. Phys. Chem., 69, 8, 2657-2667 (1965)
- E.M. Woo, L. Chang, Tacticity in Vinyl Polymers, In: Encyclopedia of Polymer Science and Technology, Wiley & Sons 2011
- D.W. van Krevelen and Klaas te Nijenhuis, Properties of Polymers, 4th Edition, Amsterdam (2009)