Steric Arrangement in Cis and Trans Configuration
Polymers with double bonds in the repeat unit give rise to different geometric isomers. For example, 1,3-butadiene can be polymerized to give poly(1,2-butadiene) or either of two geometric isomers of poly(1,4-butadiene).(2) These two isomers are called cis and trans poly(1,4-butadiene). In the case of cis-polybutadiene, the first and the forth carbon lie on the same side of the central double bond, and in the case of trans, on opposite sides of the central double bond.
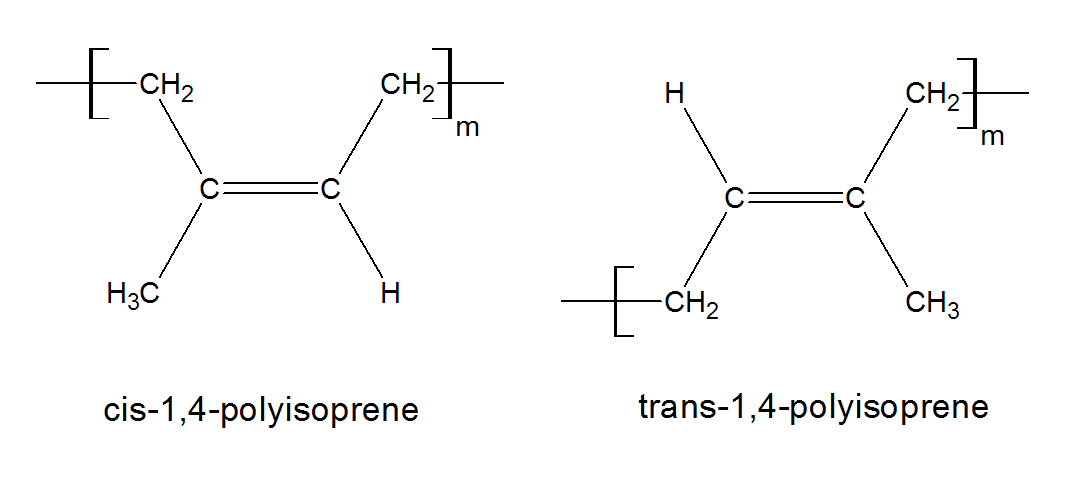
Double bonds in the cis form reduce the energy barrier for the rotation of adjacent C-C bonds compared to those in trans from, which, in turn, reduces the glass transition temperature (Tg). The affect of cis-trans isomerie on the Tg is usually not very strong, that is, the Tg shift is only about 20 K for polybutadiene. If other substituents (-Cl, -CH3 etc.) are present, the energy barrier for the rotation of adjacent bonds will increase, and hence the Tg will increase as well. Bulky substituents will also reduce the energy barrier for rotation of the trans form relative to the cis form. For example, the observed Tgs of cis and trans polyisoprene are nearly the same, whereas the Tg of the cis isomer of poly(chloro-butadiene) is about 20 K larger than the Tg of the trans isomer (see table below).
| Polymer | Tg,cis (K) | Tg,trans (K) |
| Polybutadiene | 170 (165) | 187 (190) |
| Polyisoprene | 204 | 207 |
| Poly(chlorobutadiene) | 252 | 234 |
Double bonds in the polymer backbone have a singnificant impact on the polymer properties. The double bonds in the polymer backbone prevent rotation that occurs in carbon-carbon single bonds. It is believed that the inability to rotate freely hinders the allignment of the polymer chains. Thus, unsaturation in the polymer chain reduces the crystallinity and increases the elasticity and flexibility of polymers.
References & Notes
- Robert O. Ebewele, Polymer Science and Technology, 1st Edition, 2000
- 1,2-Polybutadiene, which has a pending vinyl group, has very different properties. For example, its Tg is 260 K and more than 70 K higher than those of cis and trans 1,4-polybutadiene.