Viscosity of Polymer Solutions
Part II: Viscosity of Concentrated
Solutions
The viscosity of dilute and concetrated polymer solutions has been studied for many decades and has played an important role in understanding the effect of macromolecular structure on the dynamics of polymer solutions. Today, a huge amount of data can be found in the literature that has been analyzed by many scientists.
The relationship between viscosity and concentration depends on the polymer -solvent system, that is on the type and structure of the polymer, its molecular weight and the properties of the solvent. In general, the relationship between viscosity and concentration becomes more complicated when the polymer concentration increases, because the polymer chains start to interact with each other, first through long range hydrodynamic interactions, and then by actual contacts, entanglements, aggregates, and finally by a network of physical crosslinks. Thus, different relationships have been proposed for dilute and concentrated polymer solutions. Two common equations that are frequently used to estimate the reduced specific viscosity of polymer solutions as a function of concentration are the Huggin's and Martin's equation for dilute to moderately concentrated solutions,1,2
ηsp/c = [η] + KH [η]2 c
ηsp/c = [η] exp(KH c [η]) ⇒ ln ηsp/c = ln[η] + KH c [η]
where KH is the Huggins parameter, [η] and ηsp are the intrinsic viscosity and the specific viscosity respectively, and c is the polymer concentration, usually expressed in grams per 100 cm3 or in grams per dl. Both [η] and KH are assumed to be independent of concentration but depend on the polymer-solvent system. Thus, if KH is known, the intrinsic viscosity [η] can be calculated. However, both equations cannot be explicitly solved, meaning approximation methods have to be employed.3
The Martin and Huggins equations have been applied to a large number of systems and have given reasonable accurate estimates for the viscosity of dilute to moderately concentrated polymer solutions. For concentrated polymer solutions, Martin's equation is often expanded into a power series:
ηsp/c ≈ [η] {1 + KHc[η] + (KHc[η])2/2! + (KHc[η])3/3! + ...}
According to Matsuoka4, the higher terms describe the viscosity contributions resulting from the interactions (entanglements) between two, three, four etc. polymer coils.
Reduced Specific Viscosity versus Concentration
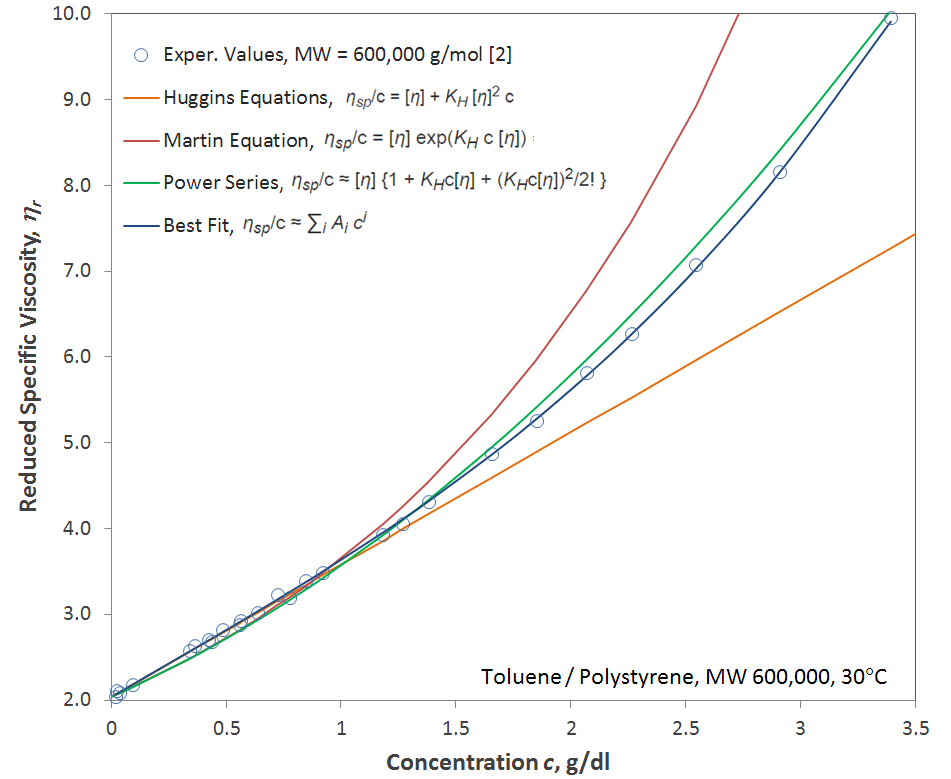
The Martin and Huggins equation are a good approximation for dilute to medium concentrated polymer solutions but are known to deviate from the experimental data at higher c[η] values whereas the power series provides a much better approximation when truncated after the third term4 (see Figure above).
Many other relationships have been developed for the viscosity of dilute and concentrated polymer solutions. One of the oldest is the Baker equation (1913):
ηsp = (1 + [η] c / n)n - 1
The parameter n depends on the polymer-solvent system but is assumed to be independent of concentration. As has been shown by Simha et al., the Baker equation can be applied over a restricted range of concentrations.2
References
- M.L. Huggins, J. Am. Chem. Soc., 64 (11), 2716 - 2718 (1942)
- S.G. Weissberg, R. Simha and S. Rothman, J. Res. Natl. Bur. Stand., 47, 4, 2257 (1951)
- M.V.S. Rao, Polymer, Vol. 34, No. 3, pp. 592-596 (1993)
- S. Matsuoka, M.K. Cowman, Polymer, 43, 3447 - 3453 (2002)