Effect of Morphology and Chain Polarity on Dielectric Properties
The dielectric properties of polymeric materials depend on the chemical composition and structure of the polymer. Most polymers are insulators, meaning these materials prevent the flow of an electrical current. Such a material is called a dielectric or an electrical insulator. Non-polar polymers with a symmetrical structure and covalent bonds are typically the best electrical insulators. Since there are no dipoles present in the repeat units, an applied field cannot cause any dipoles to allign in field direction which would weaken the electrical field, and thus increase the dielectric constant. The electrical field, however, can push the electrons slightly in the directions of the positive electrode which creates some polarization. This effect is known as electronic polarization. Since this effect is nearly instantaneous, the frequency of the electrical field will not much affect this form of polarization. Or in other words, the dielelctric constant of non-polar materials such as PE, PP, PS, PIB, and PTFE (and many other symmetric fluoropolymers) is nearly independant of the frequency. These materials also have very high resistivity.
The majority of polymers have polarized covalent bonds, that is, the electrons are drawn closer to the more electronegative atoms. These dipoles will orient themselves to lign up with the electrical field, very similar to a compass needle that attempts to lign up with the earth's magnetic field. The result is a much stronger dipole polarization and a higher relative permeability (dielectric constant) and a lower resistivity compared to non-polar polymers. The effect is known as orientational or dipole polarization.
The orientational polarization depends on the temperature and field frequency whereas the electronic polarization is more or less independent of the frequency and shows a much weaker dependency on the temperature (instantaneous polarization of non-polar plastics).
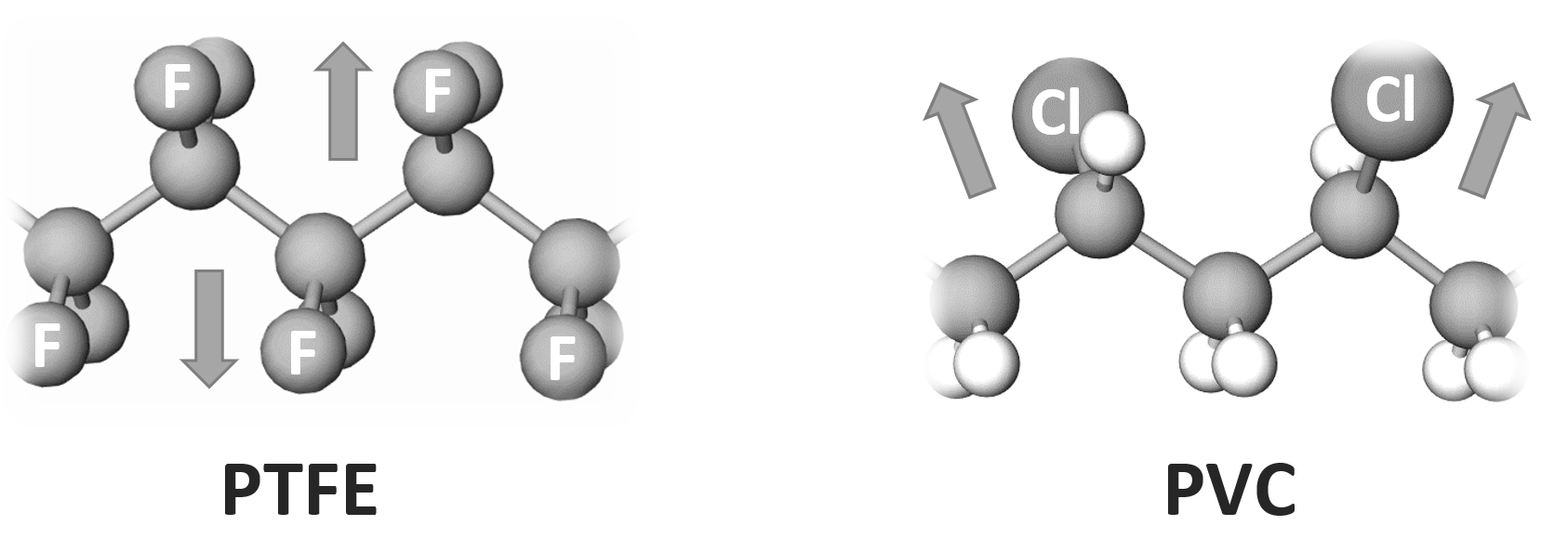
Both the orientational polarization and electronic polarization depend on the chemical composition of the polymer repeat units. For example, aromatic rings, sulfur, bromine and phenol increase the relative permittivity because they are highly polarizable. The same is true for the π bonds in aromatic rings. They are more polarizable than σ bonds, and thus also increase the relative permittivity. Very electronegative elements such as fluorine have an even stronger effect on the dielectric constant. However, this is only true if the dipole moments in the repeat units do not balance each other, as it is the case in PTFE whereas the C-F bonds in the repeat units of polyvinyl fluoride (PVF) can all allign parallel to each other and to the electric field which greatly increases the polarization. This additive effect and the strong dipole moment of the C-F bonds explains the very high dielectric constant of PVF (ε ≈ 8) whereas PTFE has a rather small dielectric constant (ε ≈ 2).
The dielectric constant is also affected by the free volume which is the volume not occupied by the molecules or repeat units of the polymer. Bulky pendant groups, which hinder polymer alignment (crystallization), increase the free volume, which in turn, decreases the dielectric constant because it decreases the number of polarizable groups per unit volume. The same is true for pores which are filled with air whose relative permittivity is about one.
Polar additives such as flame retardants, antioxidants and processing aids have usually the opposite effect, that is they increase the relative permittivity. Very effective are inorganic fillers with high dielectric constant such as carbon black and TiO2 whereas most organic compounds have only a small or modest impact on the permittivity.