Heat Capacity of Polymers
Porter (1995) has shown that the calculation of heat capacities can be simplified when using only one-dimensional Deby functions. The error incurred by this simplifications is about 1%. The calculation can be further simplified by using following approximation for the Tarasov equation:
CT = Nk/3 [ (6.7T/θ1)2 / (1 + (6.7T/θ1)2)]
The reference temperature of skeletal mode vibrations in the chain axis depend on the chain conformation. In the case of polyethylene, a pure gauche conformer chain has a reduced θ1 value by a factor of 0.575 over that of a pure trans conformer chain. The average ratio of trans and gauche states in a polymer chain can be calculated with the isomeric state theory. This is a quite cumbersome calculation. Luckily, polymers tend to show properties of the gauche and trans fractions quite separately, that is the the glass transition of gauche and trans conformers are observed separately. Furthermore, most polymers tend to be either mainly in a trans or gauche state, so that often only one value of θ1 has to be considered (usually the trans configuration).
Example: Polystyrene
The heat capacity due to the group vibrations can be calculated from the (approximate) spectrum obtained from normal mode calculations using force constants derived from infrared and Raman spectroscopy. To simplify the numerical calculation, the (quite) complicated spectum is devided into bands of approximately equal size. This approximation has only little effect on the result since the number of modes rahter than the precise frequency is important. The simplified, approximate vibrational spectrum of polystyrene is shown below.
| θ | Number | θ | Number |
| 4000 | 8 | 700 | 2 |
| 2000 | 10 | 500 | 1 |
| 1500 | 12 | 350 | 1 |
| 1000 | 8 | - | - |
Polystyrene consists of the structural units -CH2-, -CH, and -phenyl with 16 atoms per mer unit. The total number of atomic group oscillators is NE = 42 and the number of skeletal mode vibrations is N = 6. The reference temperature is θ1 = 285 K. Therefore, NE = 42 atomic group modes exist with the frequencies listed above. Each group vibration in the poystyrene chain is assumed to be an independent Einstein oscillator. Its contribution to the specific heat capacity CE can be estimated with the Einstein function:
E(θ/T) = [(θ/T)2exp(θ/T)] / [exp(θ/T) - 1]2
The sum of all 42 vibrations is the group vibration contribution to the specific heat capacity:
CE = Nk∑NE E(θ/T)
The skeletal mode contribution to the molar heat capacity can be calculated with the Tarasov equation. For polystyrene this function reads:
CT = 6 R [ (T/42.5)2 / (1 + (T/42.5)2)]
Heat capacities are usually reported at constant pressure, whereas the calculated specific heat capacities are calculated at constant volume. The thermodynamic relation between the two heat capacities is
CP - CV = -T (dV/dT)2P / (dV/dP)2T = Vα2BT
where α is the volumetric coefficient of thermal expansion, V is the molar volume of a repeat unit and B is the bulk modulus.
The (calculated) values for polystyrene are(3)
Vm = 92.8 - 99.6 cm3/mol (0 - 300K)
B = 3.58 GPa
αg = 2.37 10-4 and
αr = 5.74 10-4 and
Inserting these values in the equation above, gives corrections in the range of 0 to 6 J/(mol K). For obvious reasons the correction can be neglected at very low temperatures (below 100 K). The calculated heat capacities together with the experimental values for amorphous polystyrene reported by Gaur and Wunderlich (1981)(1) are shown below. The agreement is quite satisfactory considering the crude simplification made to calculate molar heat capacities.
Molar Heat Capacity of PS vs Temperature
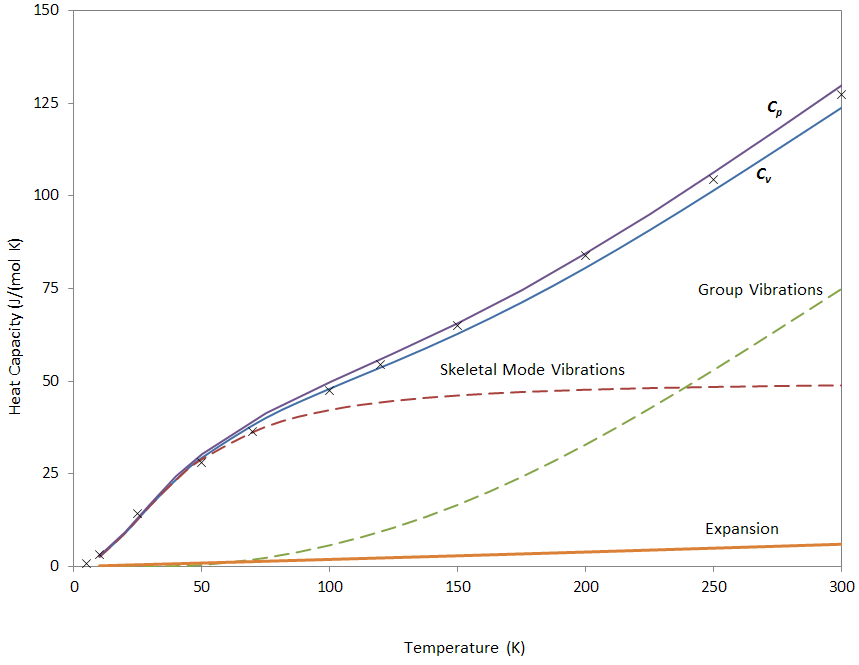
Molar Heat Capacity of PS vs Temperature
References & Notes
- U. Gaur and B. Wunderlich, J. Phys. Ref. Data, Vol. 11, No. 2, (1982)
- FD. Porter, Group Interaction Modelling of Polymer Properties, Marcel Dekker, New York (1995)
- These data have been calculated with the software 3Ps-Tg Triton Road.