Polysulfides and Polythioethers
(Polymercaptans,
Polythiols)
Properties
Polythiols also called polythioethers are compounds with mercaptane or thioether functions in the backbone. They are commonly prepared by reacting sodium sulfide with dichloro compounds:
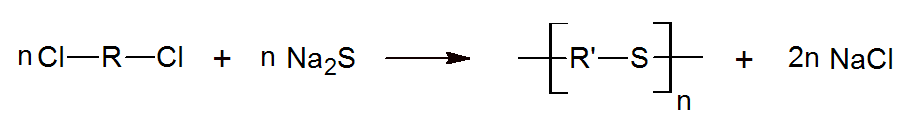
Polythiols can also be prepared by reacting dithiols with aldehydes or ketones. The reaction above is used for the commercial production of aromatic poly(mercaptanes) such as polyphenylene sulfide (PPS) which is an important engineering plastic. It has outstanding heat and chemical resistance, excellent mechanical properties and good dimensional stability. Aliphatic polythiols are also produced via the reaction above. These compounds are effective modifiers for epoxy systems and allow for fast cure. They impart excellent flexibility, impact and chemical resistance, and good anti-corrosive properties particularly when combined with other curatives such as amines or polyamines.
Aliphatic polysulfides, also known as thiokols or poly(alkylene sulfide)s, are another class of sulfur containing polymers. These compounds have two or more sulfur atoms bonded together as an anion or group in the backbone. They are often prepared by reacting sodium polysulfide with alkyl dichlorides:
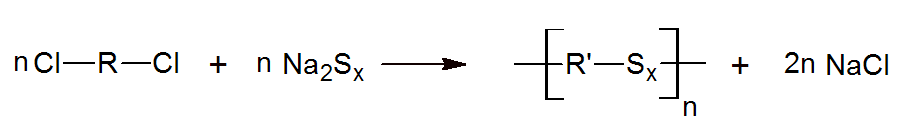
Before cross-linked, they are liquid polymers with low moisture permeability and outstanding chemical and oil resistance. Cure to high molecular weight elastomers can be achieved by oxidizing the polymer’s terminals thiol groups (-SH) to disulfide (-S-S-) links. Typical curing agents are oxygen donating compounds such as manganese dioxide, calcium dioxide, cumene hydroperoxide, and p-quinone dioxime. Other organic hydro peroxides, aldehydes and metallic paint driers can also function as curatives.
Cured polysulfides possess high flexibility, low moisture and gas
permeability, and excellent resistance to many oils and solvents
including aliphatic and aromatic hydrocarbons, ethers, ketones,
dilute acids and alkalis.
They also have excellent resistance to ozone, oxidation, sunlight, and weathering.
On the down side, they have poor thermal stability, low mechanical strength,
as well as lower resilience when compared to most other synthetic elastomers and often possess an unpleasant
odor.
The low creep resistance of the sulfur-sulfur bonds
increases the tendency to flow and relax under pressure. Thus,
polysulfides are not recommended for seals that are used under large
static and dynamic laods.
COMMERCIAL POLYTHIOETHERS, Polysulfides
- Toray (Thiokol liquid polysulfides)
- PolySpec / ITW (Polysulfide based Sealants, and Coatings)
- AkzoNobel (Thioplast liquid polysulfides)
- Chevron-Phillips (Sulfides, Disulfides)
- RheinChemie (Lubricant Additives)
- Gabriel Chemie (Polymeric Mercaptans for Epoxy)
APPLICATIONS
Polysulfides are mainly used as the base polymer for caulks, sealants, gaskets and O-rings. Polythiols may also be used as corrosion inhibitors, chain transfer agents and as a hardener for (epoxy) adhesives and coatings.
Due to their low cost, excellent chemical and weather resistance, as well as high impermeability, and good heat insulation properties, they are extensively used in building & construction, marine, aerospace, and polymer processing industries.
The service temperature of cured polysulfides is typically from -50° to 150°C with short-term exposure up to 170 °C.