Polymerization of Acrylic
and Methacrylic Esters
Poly(meth)acrylates are one of the largest volume vinyl polymers, used in countless products including paints, coatings, textiles, adhesives, elastomers, optical fibers, and shatter-resistant replacement for glass (plexigals). The primary reasons for their great popularity are their low cost, high transparency, good mechanical properties and ease of coloring and processing.
Commercial poly(meth)acrylates can be synthesized by free radical or anionic polymerization techniques with the former being the much more common method.1 Common processing methods include bulk, suspension, solution and emulsion polymerization. Free radical bulk or mass polymerization is the principal method for producing high quality acrylic glass sheets, such as Plexiglas. The reaction mass consists mainly of polymerizable compounds which polymerize under carefully controlled conditions without or with only small amounts of polymerization catalysts. A major drawback of bulk polymerization is poor heat transfer, particularly in the later stages of the polymerization when the viscosity is high. Thus, runaway reaction can easily occur since the reactor temperature is very difficult to control which also can lead to a non-uniform product. For these reasons, most acrylic resin products are produced by using one of the other polymerization processes. For example, emulsion and solvent polymerization are frequently used to produce water- and solvent-based acrylic resins which find widespread use as adhesives and paint/coating resins, whereas suspension (and in some cases solvent-based) polymerization is frequently employed to produce beads and powders which then can be molded to numerous plastic products. In all of the three heterogenous processes, solvent or water acts as a diluent or dispersion medium and aids in the transfer of heat of polymerization.
A major drawback of (meth)acrylate monomers is their high sensitivity towards oxygen which can inhibit (or stop) polymerization, particularly at the air-monomer interface. To prevent inhibition by dissolved oxygen, the (meth)acrylic monomers must be completely degassed before polymerization.10
Acrylates are one of the few types of vinyl monomers that can be polymerized at moderate temperatures without the addition of free radical initiator. The process is known as spontaneous thermal polymerization and has been extensively studied for more than half a century.2,3 However, until recently, there was no consensus about the correct mechanism. The first plausible mechanism was suggested by Flory.2 He postulated that two vinyl monomers (M) combine to form a 1,4-diradical (M2··), which either ring-closes to form cyclobutane dimer (CBD) or reacts with a third vinyl monomer to produce two monoradicals (M·, M2·) via hydrogen transfer or abstraction.
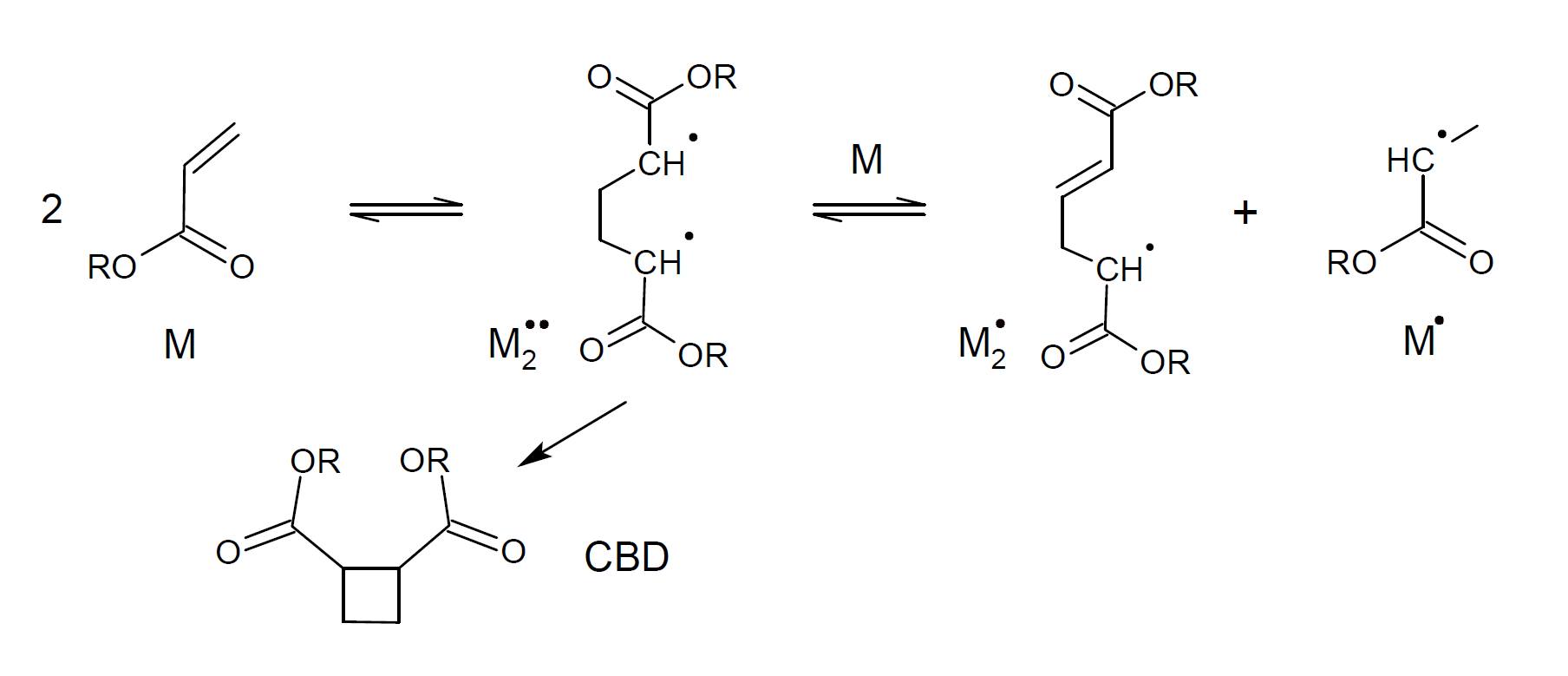
Another mechanism of self-initiation was suggested by Mayo.3 He postulated that two monomers combine to form a Diels-Alder vinyl dimer (DAA), which then transfers a hydrogen to a third monomer (molecular assisted homolysis) to form two monoradicals (DAA·, M·).
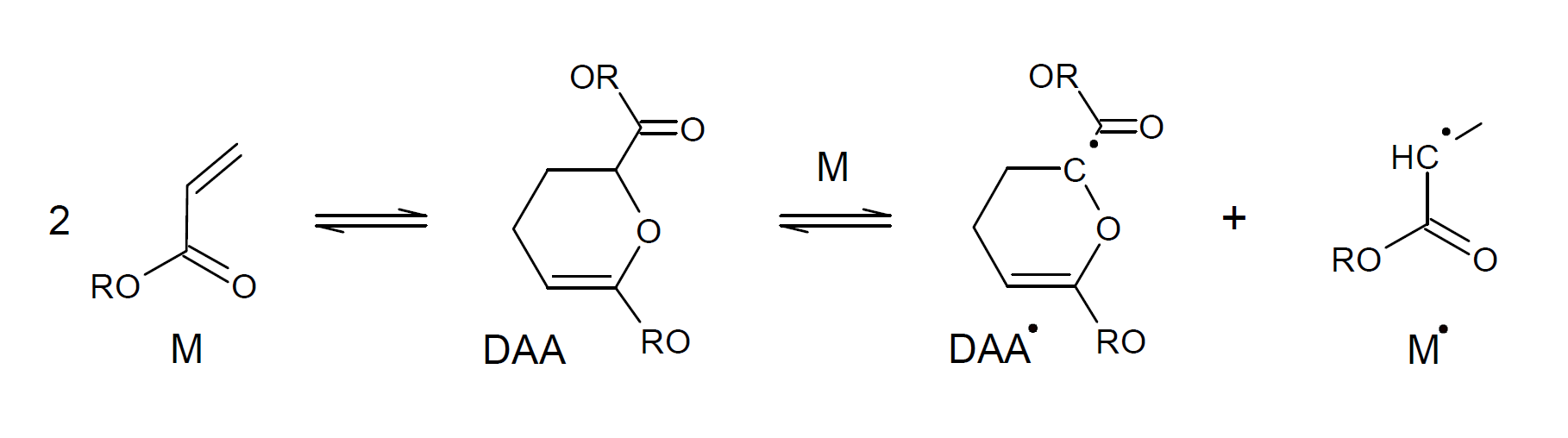
The spontaneous thermal self-initiation of acrylates has been extensively studied by Srinivasan et al. (2010).4 They showed that the diradical moiety is the one that generates monoradical species for initiating polymerization of acrylic monomers. The absence of Diels-Alder adducts and the involvement of diradical species in initiation of spontaneous thermal polymerization of MMA supports this postulate, i.e. that the Flory mechanism is indeed the mechanism for self-initiation of (meth)acrylate polymerization.5
Heat induced polymerization is relative slow and has to be conducted at relative high temperatures.12,13,14 To speed up the polymerization process and to lower the reaction temperature, free radical initiators such as peroxides, or azo compounds are often added to the monomer (mixture). These compounds decompose thermally or by UV irradiation to produce free radicals. The mechanism of free radical polymerization of acrylates is shown below. In a first step, a radical (I·) reacts with an acrylic monomer (Initiation).6 A radical will typically attack the less substituted carbon atom of the (meth)acrylate molecule because a carbon radical is more stable when it is located at the more substituted carbon due to induction and hyperconjugation (Anti-Markovnikov rule). In other words, the more stable radical intermediate is the one with the more substituted carbon. After a carbon radical is formed, it will attack the double bond of a nearby (meth)acrylate monomer which in turn reacts with another monomer to form a trimer and so on. The progressive addition of monomers to the growing polymer chain is called propagation. It typically leads to a head-to-tail arrangement which is the normal sequence in free radical propagation. (The carbon atom with the most substituents is usually called the head.)
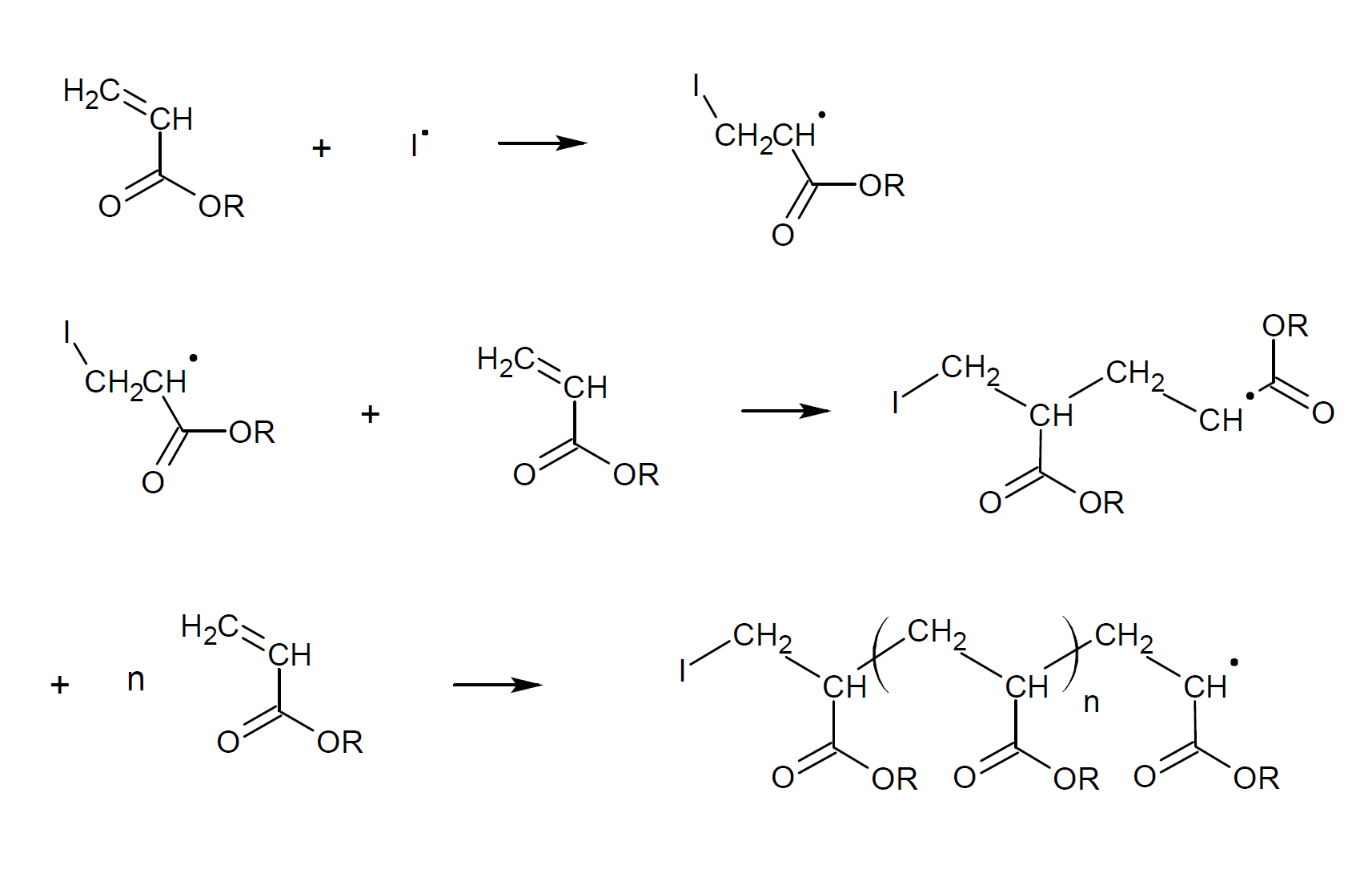
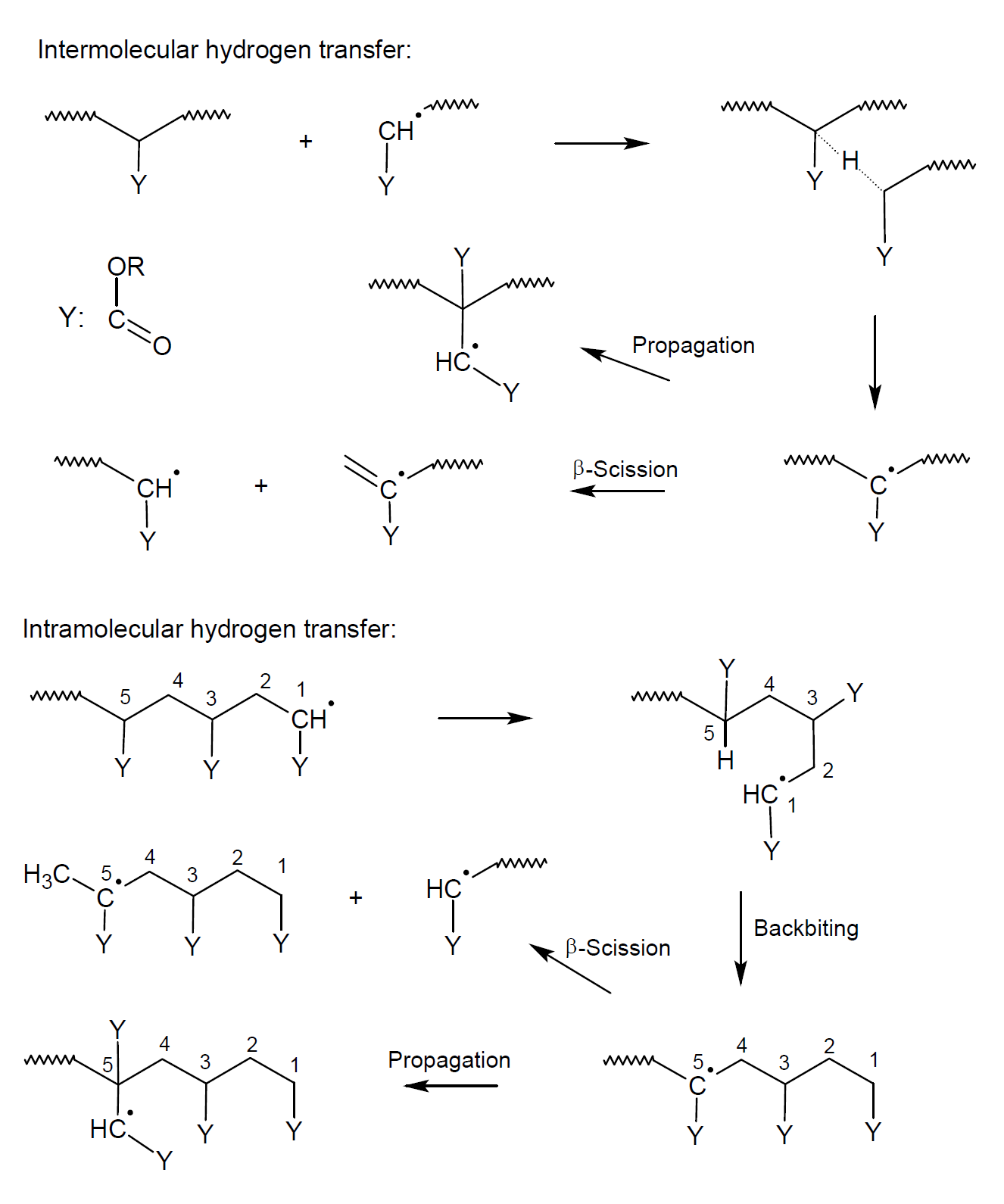
The reaction is exotherm and exhibits rapid rate acceleration, and thus the monomer-polymer mixture has to be cooled. It typically results in the formation of very high-molecular weight polymers. In many cases, some crosslinking, branching, and head-to-head arrangement is observed. The latter is the result of chain termination reactions whereas branching and crosslinking is the result of back-biting reactions (intermolecular chain transfer) and hydrogen abstraction from nearby chains (intramoelcular chain transfer) giving mid-chain radicals (see below). The contribution of backbiting and intramoelcular chain transfer reactions increases with increasing temperature and decreasing radical concentration giving more mid-chain radicals and branching.9
The two most common termination reactions in free-radical polymerization are recombination (coupling) and disproportionation (hydrogen atom transfer with simultaneous formation of a double bond).
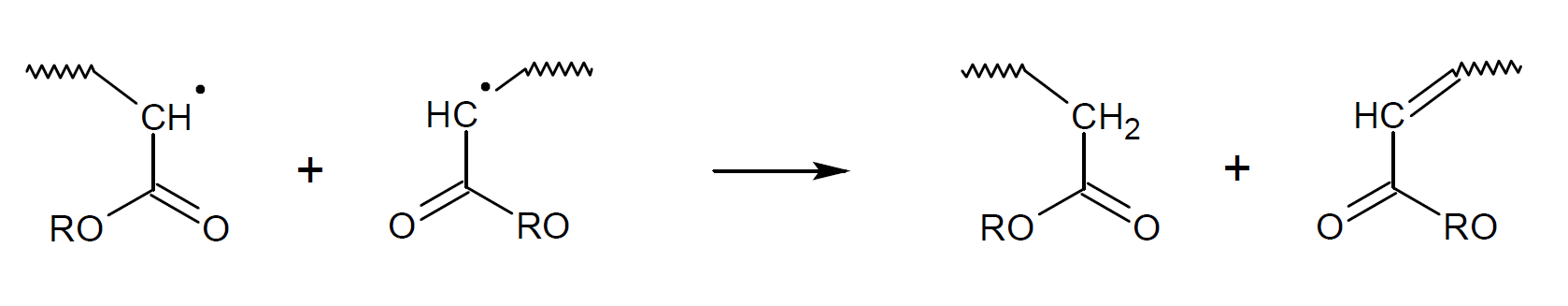
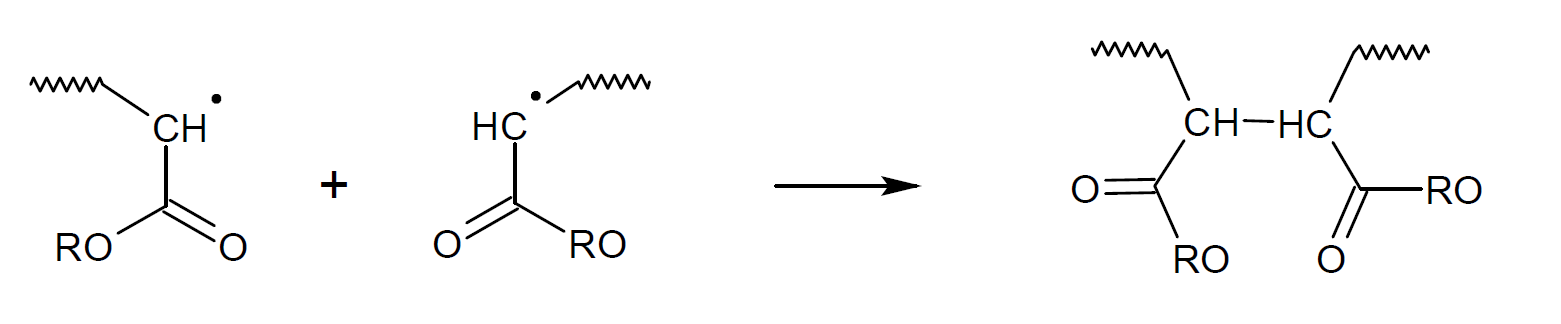
In the case of methyl (meth)acrylate, disproportionation dominates at low temperatures,7-9 whereas in the case of styrene recombination is more favorable.8 The same is true for most other methacrylic and acrylic monomers with other (bulky) side groups because the bond becomes more strained due to the two bulky groups in close proximity to each other. Recombination is also less likely to occur due to increased steric hindrance. The contribution of recombination typically increases at higher temperature because recombination is enthalpically and entropically less favored than disproportionation (le Chatelier's principle11).
References and Notes
Anionic polymerization is not widely used on an industrial scale because the monomers must be very pure and the polymerization has to be carried out at very low temperatures.
P. J. Flory, J. Am. Chem. Soc., 59, 241-253 (1937)
F. R. Mayo, J. Am. Chem. Soc., 90, 1289-1295 (1968) & 75, 6133-6141 (1953)
S. Srinivasan, M.W. Lee, M.C. Grady, M. Soroush, A.M. Rappe, J. Phys. Chem. A, 114, 30 (2010)
J. Lingnau and G. Meyerhoff, Polymer, 24, 11, 1473-1478 (1983)
Not all radicals form polymer radicals; some react with each other to form inactive species.
J.C. Bevington H.W. Melville R.P. Taylor, J. Poly. Sci., 12, 1, 449-459 (1954)
Y. Nakamura and S. Yamago, Macromolecules 48, 18, 6450-6456 (2015)
Y. Nakamura, R. Lee, M.L. Coote & S. Yamago, Macromol. Rapid Commun. 37, 506-513 (2016)
Oxygen (air) is much more effective in inhibiting polymerization of alkyl methacrylates than alkyl acrylates. Nevertheless, dissolved oxygen should be removed from both types of monomers because even small amounts of oxygen affect the rate of polymerization.
The Le Chatelier's principle states, if a system in a dynamic equilibrium is subjected to change in concentration, temperature, volume, or pressure, the equilibrium position of the system shifts to counteract the disturbance.
Fenouillot et al. attributed high polymerization rates at elevated temperatures to trace amounts of initiating impurities, while a second, slower polymerization stage could be considered as the actual thermal polymerization. They also found that small amounts of chain transfer agents can initiate MMA polymerization at high temperatures.13
F. Fenouillot, J. Terrisse & T. Rimlinger, Int. Poly. Proces., 13: (2), pp. 154-161 (1998)
H. Riazi, A.A. Shamsabadi, P. Corcoran, M.C. Grady, A.M. Rappe and M. Soroush, Processes 6, 3 (2018)