Chain Transfer
Chain transfer is a termination reaction by which the free-radical site of a growing polymer chain is transferred to another molecule:
Rr· + S → Pr + S·
The newly formed solvent or monomer radicals may then reinitiate chain growth by resumption or propagation reactions:
S· + M → R1·
R1· + M → R2 etc.
The amount of chain transfer to monomers is usually low because this reaction requires breaking strong carbon-hydrogen bonds. However, there are some exception. For example, vinyl chloride has a rather large chain transfer constant when compared to most other vinyl monomers. The same is true for chlorinated solvents like chloroform and ethylene dichloride. In many cases, the transfer of the radical takes place through the removal of an atom (H, Cl, Br etc.). The reactivity depends on the bond strength between the labile atom that is abstracted and the rest of the molecule. In general, the reactivity of a chain transfer increases with increasing substitution at the α-carbon. For example, for vinyl monomers such as styrene or methyl methacrylate the chain transfer increases in the order
Benzene < Toluene < Ethyl benzene < Isopropyl benzene
Common chain transfer agents are halogen compounds, like chloroform and carbon tetrachloride, certain aromatic hydrocarbons, and thiols (mercaptans). These compounds are often called modifiers or regulators. Very strong chain transfer agents like pentaphenyl ethane, carbon tetrabromide, and certain mercaptanes, even when added in small amounts, will have a strong effect on the final molecular weight, that is, with increasing amount of regulator, the degree of polymerization sharply decreases.
Chain transfer can also occur within a poymer (intermolecular chain transfer), for example from the terminal group to a location on the polymer backbone by hydrogen abstraction. This reaction is known as backbiting. The result is branching of the backbone. In the case of polyethylene (see figure below), this reaction leads to short branches of about four to five repeat units.
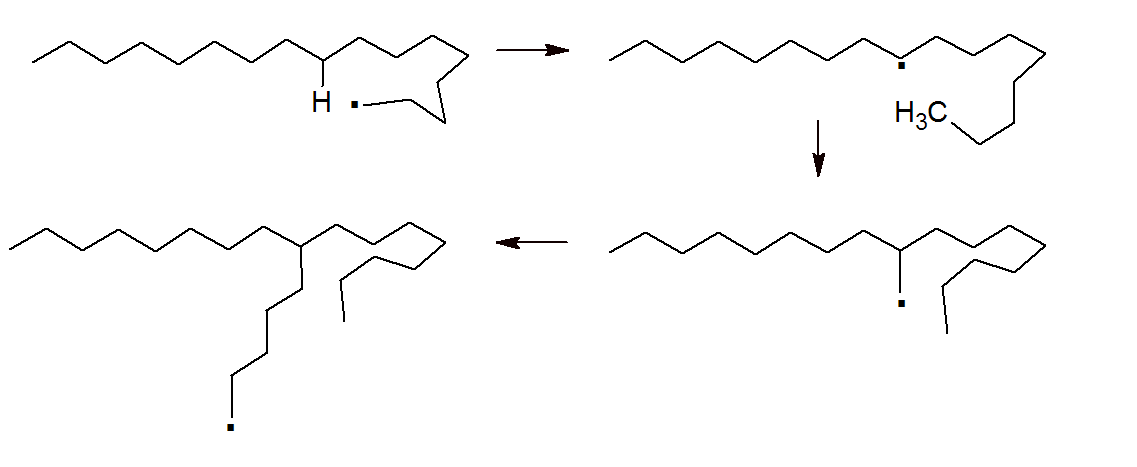
Chain transfer not only depends on the type of transfer agent but also on the temperature and polymer type. An increase in temperature almost always increases the amount of chain transfer, thus lowers the molecular weight2, whereas the viscosity (i.e. polymer concentration, degree of polymerization) and polymerization rate2 have no or only little effect on the rate of chain transfer.
Notes
- This is also true when the molecular weight is controlled by chain termination because termination increases with increasing temperature as well.
- An exception is transfer to an initiator. In this case, the rate of chain transfer increases rapidly with the rate polymerization.