Azeotropic Copolymerization
Copolymers constitute a large portion of commercial polymers. The monomers can have similar of very different physical properties, which results in a large variety of copolymers with very different properties and end uses.
The incremental composition of the copolymers (i.e. the compostion along the backbone) will usually vary, because the monomers have different reactivites; to be more specific, the composition of the copolymer will depend on the reactivity ratios:
r1 = k11 / k12
r2 = k22 / k21
where kxy are the reaction constants of the four different types of polymerization reactions:
M1* + M1 → M1M1* R11 = k11 [M1*] [M1]
M1* + M2 → M2M1* R12 = k12 [M1*] [M2]
M2* + M2 → M2M2* R22 = k22 [M2*] [M2]
M2* + M1 → M1M2* R21 = k21 [M2*·] [M1]
where M1* and M2* represent
chains terminating in a unit M1 and M2.
Both the composition
of the monomer blend f1
and the composition of the copolymer increments F1 (usually expressed as mole fractions) will vary throughout the
polymerization. Thus, the polymer produced over the range of
conversion will vary in
composition along the backbone.
The figure below shows the incremental polymer composition as a function of monomer composition for different reactivity ratios. If the reactivity ratios of the two monomers are both either less or greater than unity, the curves cross the straight line f1 = F1. The point of interception is the so called azeotropic blend. In analogy to vapor-liquid equilibria, the composition of an azeotropic blend will be constant throughout the polymerization, that is, both the feed and the copolymer composition will not change.
Incremental Polymer Composition as a Function of Monomer Composition for Different Reactivity Ratios
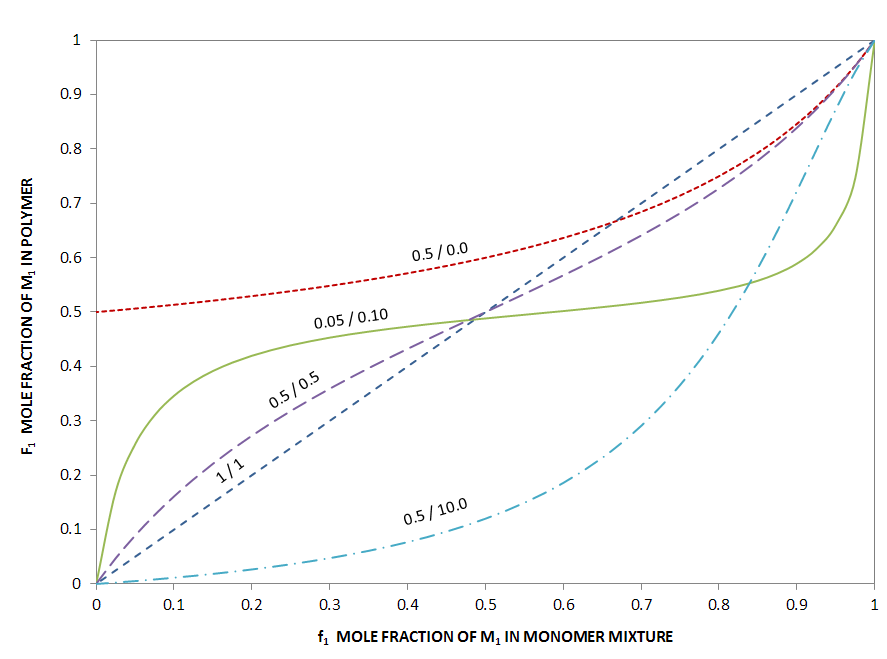
The condition for azeotropic monomer blends can be calculated with the Mayo-Lewis equation:
d[M1]/d[M2] = (|M1|/|M2|) · (r1[M1]/[M2] + 1) / ([M1]/[M2] + r2)
With d[M1]/d[M2] = (|M1|/|M2|) this equation reads
[M1] / [M2] = (1 - r2) / (1 - r1)
f1,c = (1 - r2) / (2 - r1 - r2 )
If both reactivity ratios are smaller or greater than unity, then f1,c has non-negative solution and an azeotropic blend exist. However, if r1 > 1 and r2 < 1 or r1 < 1 and r2 > 1, then no critical blend exist.