Copolymerization of
Vinyl Polymers
Copolymers constitute a large portion of commercial polymers. They vary in both the composition and distribution of the different monomers along the backbone. The monomers can have similar of very different physical properties, which results in a large variety of copolymers with very different properties and end uses. For example, small amounts of dienes or trifunctional monomers can be introduced into an aliphatic polymer to allow for crosslinking of the polymer to achieve certain mechanical properties.
Blending two monomers results in four simultaneously occuring propagation reactions with different rate equations:
M1· + M1 → M1· R11 = k11 [ M1·] [ M1]
M1· + M2 → M2· R12 = k12 [ M1·] [ M2]
M2· + M2 → M2· R22 = k22 [ M2·] [ M2]
M2· + M1 → M1· R21 = k21 [ M2·] [ M1]
where M1· and M2· represent chain
radicals of type 1 and 2, that is, the chains terminal, free radical
bearing unit is a monomer of type 1 and 2.
If the polymer chains are sufficiently long,
both initiation and termination are exceedingly rare events.
Furthermore, at a steady state, the generation and elimination of
radicals are equal. Then we have
k21 [M2·] M1] = k12 [M1·] [M2]
The rates of monomer consumption are given by
d[M1]/dt = k11 [M1·] [M1] + k21 [M2·] [M1]
d[M2]/dt = k22 [M2·] [M2] + k12 [M1·] [M2]
To eliminate the radical concentration, we divide the first equation by the second equation. We then obtain the Mayo-Lewis1 equation:
d[M1]/d[M2] = (|M1|/|M2|) · (r1[M1]/[M2] + 1) / ([M1]/[M2] + r2)
where r1 and r2 are monomer reactivity ratios defined by
r1 = k11 / k12
r2 = k22 / k21
To predict the composition of a copolymer based on the reactivity ratios, we introduce two more variables: the mole fraction of unreacted monomer f1 and the mole fraction in an increment of copolymer F1 formed at a given stage in the polymerization process, also called instantaneous composition:
f1 = [M1] / ([M1] + [M2]) = 1 - f2
F1 =(r1f12 + f1f2) / (r1f12 + 2f1f2 + r2f22)
By definition, r1 and r2 describe the relative preference of a certain radical to add to its own monomer in the growing polymer chain. If, for example, r1 is greater than one, then the radical of type 1 has a preference to react with a monomer of same kind and if r1 is smaller than one, then it is more likely that it reacts with a monomer of the other kind. If the two types of radicals have different selectivities in their choice of monomer, then r1 ≠ r2 or
r1 r2 = k11 k22 / k12 k21 ≠ 1
The quantity r1 r2 is the ratio of the product of the rate constants for reactions of a radical with a monomer of same kind to the product of the rate constants for the cross reactions.
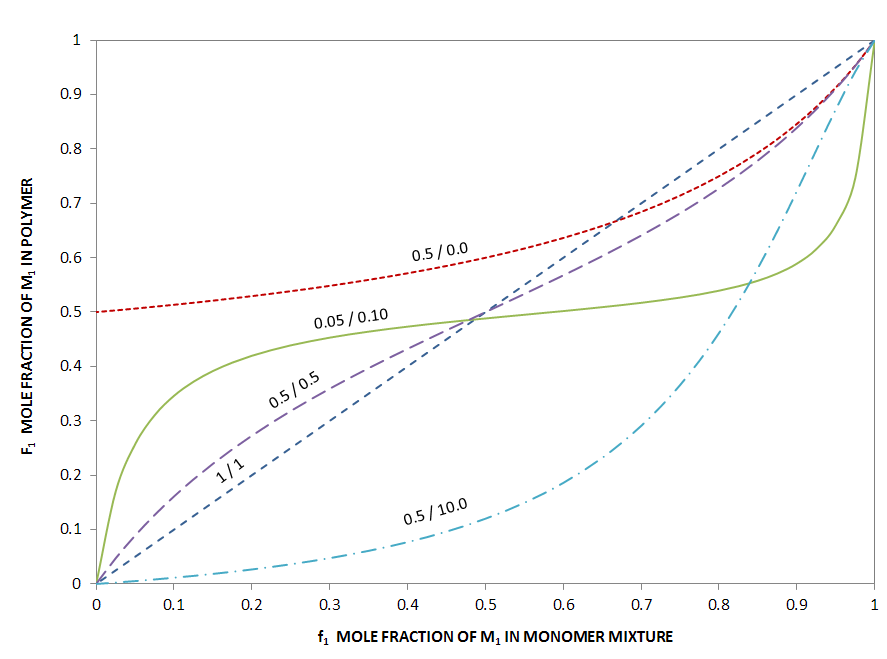
The figure below shows the incremental polymer composition as a function of monomer composition for different reactivity ratios. If the product of the reactivity ratios r1 r2 is much larger than one, the reaction of a radical with a monomer of the other kind is a very unlikely event. The two curves with the lowest inital slope are examples of this case (r1/r2 = 0.5/10 andr r1/r2 = 0.5/0.0). In such copolymers, sequences of like monomers should exist in much greater abundance than in a random copolymer. The case r1 = 1/r2 represents the ideal case; each radical has no preference and the copolymer has a completely random sequence of monomers. For the special case of equal reactivity, r1 = r2 = 1, the sequence is not only random, but the composition is identical with the compsition of the monomer blend. This is one example for a monomer blend composition that does not change during polymerization. It is refered to as an azeotropic mixture in analogy to vapor-liquid equilibria.
Incremental Polymer Composition as a Function of Monomer Composition for Different Reactivity Ratios
Copolymerization may therefore be classified according to the monomer sequence. Two extreme cases are sequences of alternating and blocked arrangements of monomers. The former is only possible if the two monomers M1 and M2 are unable to hompolymerize or when they have a strong preference to react with each other, and the latter occurs when the two monomers have a very low probability of cross-polymerization or when they are polymerized by controlled sequential monomer addition. Two other common types of copolymers are gradient and statistical (random) copolymers. Gradient copolymers have a varying composition along the polymer chain. They are produced when the monomers M1 and M2 have very different reactivities or when the composition of the monomer feed is deliberately gradually changed. This method is called gradient copolymerization. Statistical copolymers, on the other hand, are produced when the homo- and cross-propagation rates of the two monomers M1 and M2 are very similar or when the monomer feed ratio is kept constant throughout the polymerization.
References
- F.R. Mayo, F.M. Lewis, J. Am. Chem. Soc. 66, 1594 (1944)
- F.R. Mayo and C. Walling, Chem. Revs., 46, 191 (1950)
- F.T. Wall, J. Am. Chem. Soc., 66, 2050 (1944)
- Paul L. Flory, Principles of Polymer Chemistry, Ithaca, New york, 1953
- R. Simha and L.A. Wall, J. Res. Natl. Bur. Stand., 41, 5, 521 (1948)
Revised November 23, 2019