Isoprenoids (Terpenoids)
and Derivatives (Polyterpenes)
Properties
Isoprenoids (also called terpenoids) are naturally occurring organic compounds made up of isoprene units.1 They are the largest group of natural occurring hydrocarbons with well over 30,000 known compounds and one of the most structural diverse classes of natural resins. This group of hydrocarbons was named terpenes2 because they are extracted from terpentine oil which is harvested from wood sources and from citrus fruits. Terpenoids consist of C = 5, 10, 15, 20..., n > 40 carbon units and are classified as hemiterpenes (C5), monoterpenes (C10), diterpenes (C20), triterpenes (C30), tetraterpenes or carotenoids (C40), and polyterpenes (Cn, n > 40). The three most important commercial terpenes are α-pinene, β-pinene and d-limonene. The pinenes are derived mainly from two processes: (a) pine stump extraction with subsequent steam distillation leading to wood turpentine and (b) kraft sulfate pulping process when pulping pine trees leading to sulfate turpentine. d-Limonene is obtained as a by-product in the extraction of citrus juices whereas l-Limonene is found in the needles and cones of pine trees. d-Limonen has a strong lemon-like odor and is is used in fragrances and food flavours.
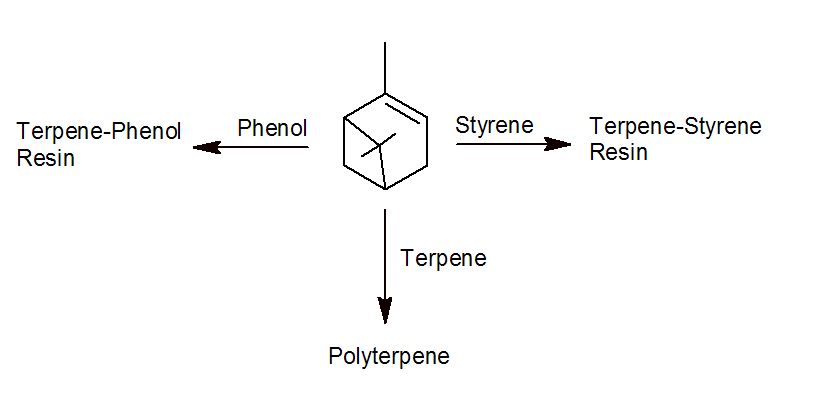
For commercial resin applications, terpenes are usually further modified or polymerized. The three most important types of commercial terpene resins are pinene-based polyterpene and styrene- and phenol-modified polyterpene. The terpene phenolic resins have a relative high softening point of about 100 - 160°C due to their higher polarity whereas the softening point of (unmodified) polyterpenes greatly depends on the molecular weight. It typically varies from about 10 to 135°C. Unmodified polyterpenes are highly compatible with polyolefins and the midblock of SIS and SBS block copolymers. They impart excellent tack and peel adhesion when blended with these polymers. The phenolic and styrenic terpenes, on the other hand, are (highly) compatible with the end blocks of SIS and SBS block copolymers and with any polymer of higher polarity such as EVA, PUR, SBC, and acrylics.
Synthesis
Polyterpenes are typically synthesized by cationic polymerization using a suitable solvent and Lewis acid catalyst. Depending on the temperature, catalyst, and composition, cationic chain growth yields low to high molecular weight unsaturated polyterpenes:3-4 To improve the color and heat stability, they are often (partially) hydrogenated.
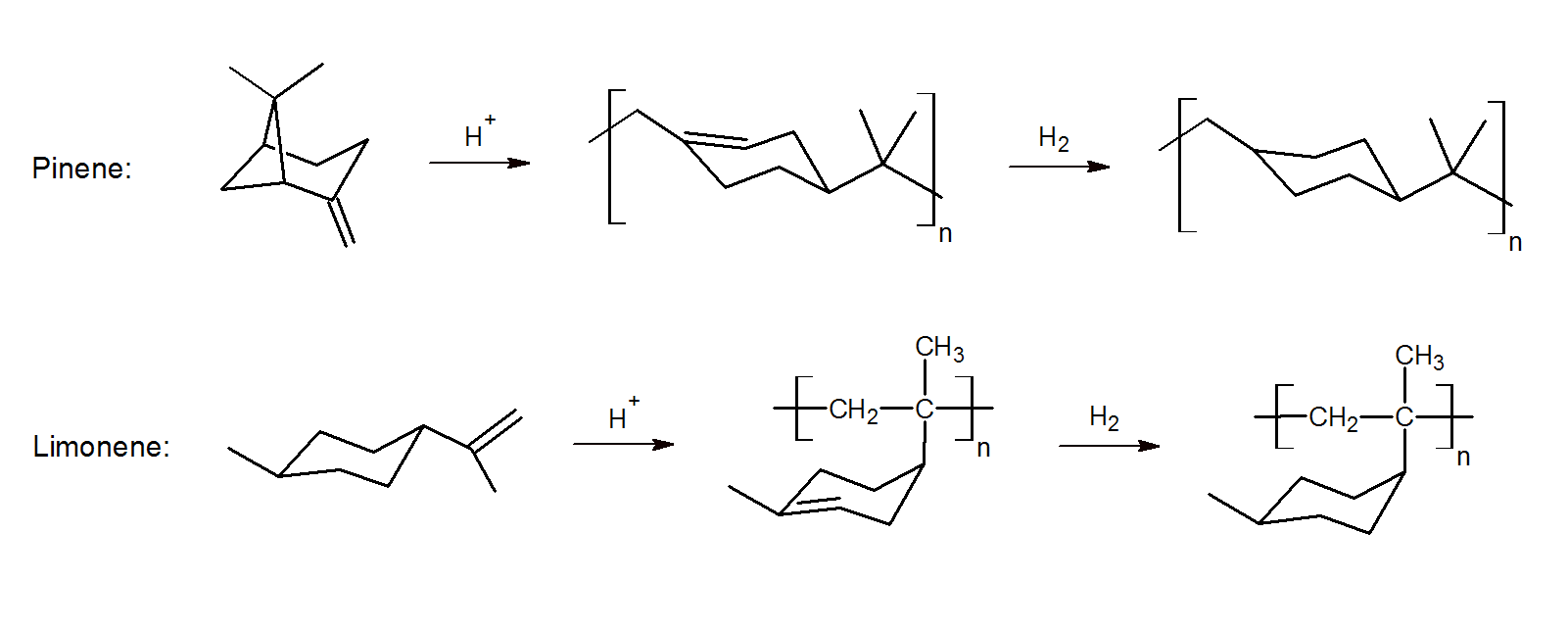
Free radical polymerization of (modified) pinenes and limonene has also been reported.5 The alpha-pinene is first transformed into pinocarvone which possesses a reactive exo-methylene group. The polymer can be polymerized in fluoroalcohols by selective ring-opening radical polymerization. The resulting high molecular weight polymers have excellent thermal properties and a high glass transition temperature.
COMMERCIAL Terpene Products
Terpenes and their derivatives are commercially available in large quantities. Major manufacturers and suppliers of these resins are Foreverest, DRT, Formosa, Kraton, Pinova, Yasuhara Chemicals, and Arakawa Chemicals among several other companies.
APPLICATIONS
Polyterpenes are frequently used as (co-)tackifiers in many high-quality hotmelt and pressure sensitive adhesives. They also find (limited) use as performance modifiers, homogenizing agents and adhesion promoters in paints, varnishes, detergents, sealants, caulks, and rubber compounds. They are very universal tackifiers and modifiers because they are compatible with many common elastomers including natural rubber (polyisoprene), EVA, SBR, SIS, SBS, acrylics and polyolefins. Many grades meet a broad range of FDA requirements, and are approved for direct food contact. However, they are more expensive than rosin and hydrocarbon resins and are therefore used on a much smaller scale.
Styrenated terpenes are a good choice for high-quality adhesives due to their light color, low odor, and outstanding resistance to oxidation and discoloration. The combination of aromatic and aliphatic groups in the polymer results in excellent peel adhesion to many substrates. These resins are compatible with a very broad range of elastomers including high and low vinyl acetate EVA, acrylics, SBR, SIS, and SBS.
Aromatic modified polyterpenes and terpene phenolic resins possess excellent compatibility with high polarity polymers.They impart aggressive hot-tack and adhesion to difficult-to-bond substrates such as coated and recycled paper, glass and metal foil and are for this reason frequently used as (co-)tackifiers in book binding and packaging hot melt and PSA adhesives.
1The most important commercial isoprenoid is natural rubber, a polymer that consists predominantly of isoprene repreat units in which the double bonds are all cis. The polymer is of such importance that it is discussed in detail separately.1
2The derivatives are strictly speaking not terpenes but terpenoids.
3H. Nakajima, P. Dijkstra and K. Loos, Polymers, 9, 523 (2017)
4S. Sharma, A.K. Srivastava, Ind. J. Chem. Technol., 12, 62-67 (2005)
5H. Miyaji, K. Satoh, M Kamigaito, Angewandte Chemie, 128, 4, 1394-1398 (2016)