Free Radical Polymerization
of Vinyl Monomers
Free radical polymerization (FRP) is one of the most important synthesis routes for obtaining vinyl polymers. The relatively non-specific nature of the free radicals towards vinyl and other unsaturated monomers makes FRP one of the most versatile polymerization methods. For example, 40 to 45 percent of all polymers and synthetic rubbers are produced via a free radical polymerization process.1,2 This includes polystyrene, poly(methyl methacrylate), polyvinyl acetate, polyvinyl chloride, polybutadiene, polychloroprene and polyethylene among many other large volume polymers and elastomers.2
The polymerization starts off with a molecule called initiator.4 Two very common initiators are benzoyl peroxide (BPO) and 2,2'-azo-bis-isobutyrylnitrile (AIBN). Both molecules have a strong tendency to fall apart in a rather unusual way; that is, the pair of electrons in the bond which is broken, will separate. The two fragments with unpaired electrons are called free radical initiators. Following their generation, the free radicals react with a monomer thereby creating a new radical that can start a chain growth polymerization:
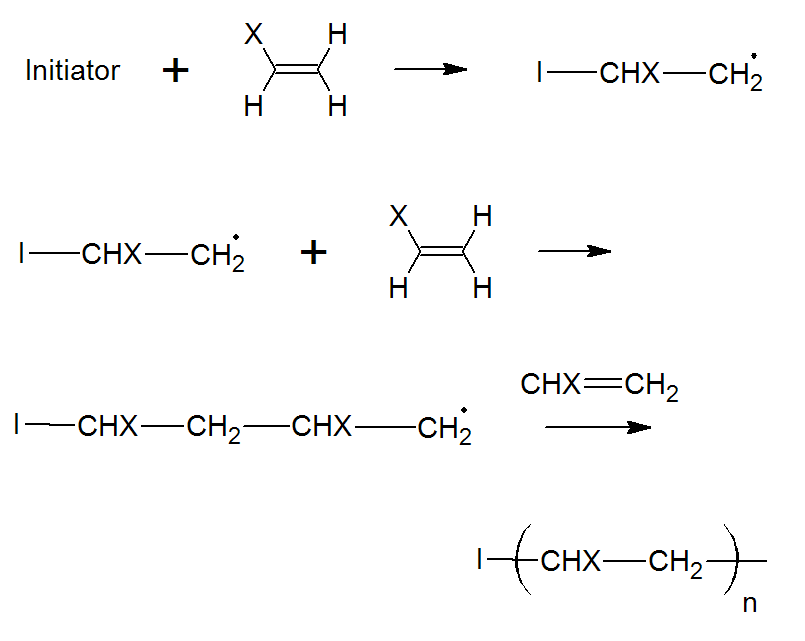
where X is a substituent which could be any of the following: C6H5, Cl, Br, OCOCH3, COOR or H. The mechanism also includes disubstituted monomers such as vinylidene chloride and methyl methacrylate.
A common feature of the vinyl polymerization is that the active center of the growing chain is retained by a single polymer molecule throughout the course of its growth. Thus the partially polymerized mixture consists of high molecular weight polymers and unchanged monomers with virtually no chains of intermediate molecular weight. In fact, polymers formed during the early stage of polymerization, even during the first fraction of a percentage conversion, are comparable in molecular weight to those formed at the advanced stage of the process.
Most vinyl monomers are readily susceptible to catalysts and photo-activation. To prevent uncontrolled or unwanted polymerization, inhibitors are usually added to the monomer. These compounds are extremely effective and can prevent polymerization even at very low concentration at or below 10-4 molar.3
References & Notes
Dietrich Braun, Origins and Development of Initiation of Free Radical Polymerization Processes, Int. J. of Poly. Sci. Vol. 2009, e893234/1-10
Peter Nesvadba, Radical Polymerization in Industry, in Encyclopedia of Radicals in Chemistry, Biology and Materials, John Wiley & Sons 2012
Paul L. Flory, Principles of Polymer Chemistry, Cornell University Press, Ithaca, New york, 1953
The initiators are sometimes erroneously called catalysts. Initiators are consumed in the reaction while catalysts are regenerated after the completion of the reaction.
-
Alfred Rudin and Phillip Choi, The Elements of Polymer Science & Engineering, Academic Press, Oxfors 2013