Autoacceleration; Trommsdorff or Gel Effect
In free-radical
polymerizations, an autoacceleration of polymerization rate is
typically observed when the monomer concentrations is high and when
the conversion reaches a certain value. For free-radical polymerization one expects a first-order kinetic with
respect to the monomer concentration. This is indeed observed for most vinyl polymers over a wide extent of polymerization. However, the polymerization of some monomers, both undiluted and
diluted,
shows a marked deviation from first-order kinetics at a certain
conversion. One observes a considerable increase in both the
polymerization rate and the molecular weight which is known as the
Trommsdorff or Norish-Smith effect in recognition
of their contributions in this field. The effect is particularly pronounced with methyl
methacrylate, methyl acrylate, and acrylic acid at various
concentrations. It occurs also with other monomers, such as styrenes
and vinyl acetate, but for these monomers the effect is less
pronounced.
Autoacceleration is independent of the initiator and
can be observed even under isothermal conditions. In fact, if the
reaction is exotherm, autoacceleration results in in a noticeable
increase in temperature. An example is shown below for the
polymerization of methyl methacrylate at 50 °C in the presence of
benzoyl peroxide initiator (BPO) at various initial concentrations of
monomer in benzene.3
Polymerization of PMMA at 50 °C in the presence of BPO at various Concentrations of Monomer in Benzene
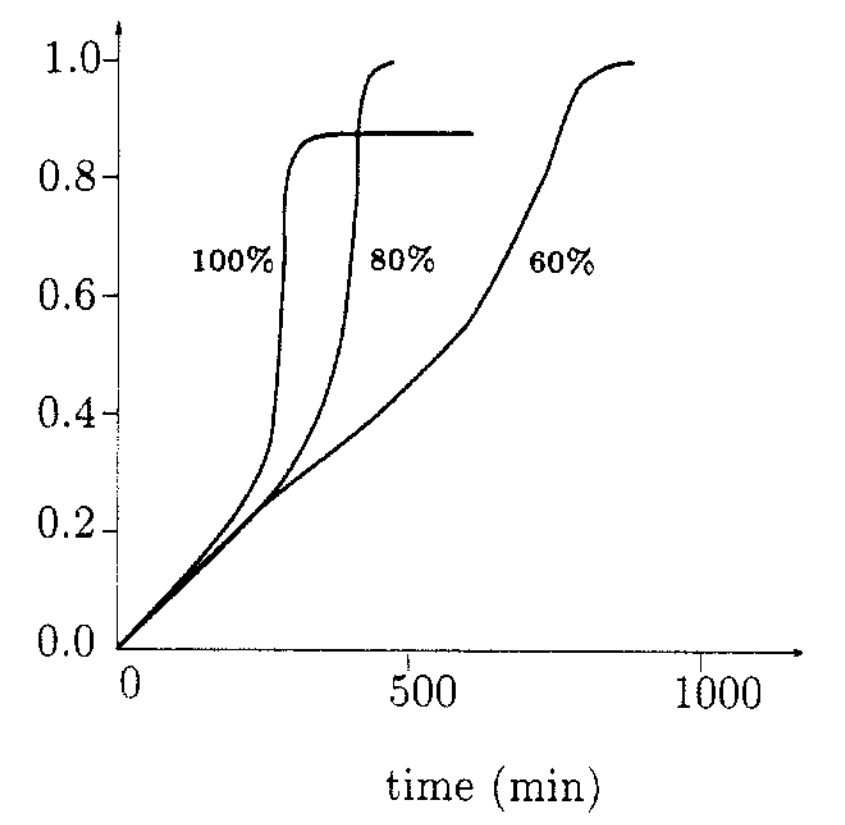
According to the kinetics of free radical polymerization, the rate of polymerization depends on the rate constants of initiation, propagation and termination:
Rp ∝ kp (f kd / kt)1/2
Since the effect is not a function of the initiator, it must depend on the
rate constant ratio kp/kt1/2, which has to increase by as much as a hundredfold to explain the effect shown in the figure above.
Norrish and Smith1, Trommsdorff2, and Schulz and Harborth3
postulated that the drastic increase in the rate of polymerization
and the simultaneous increase in the average molecular weight is
caused by a noticeable decrease in the termination rate when the system reaches a certain concentration and molecular weight. They attributed the decrease in the termination rate
kt
to the high viscosity of the medium at high(er) conversion rates
(around 20 %).
According to Trommsdorff et al., the overall diffusion rate of the
growing polymer chains depends on the viscosity of the medium. If
the viscosity is high, the termination rate, that is, the combination of
two free chain radicals, becomes diffusion controlled.
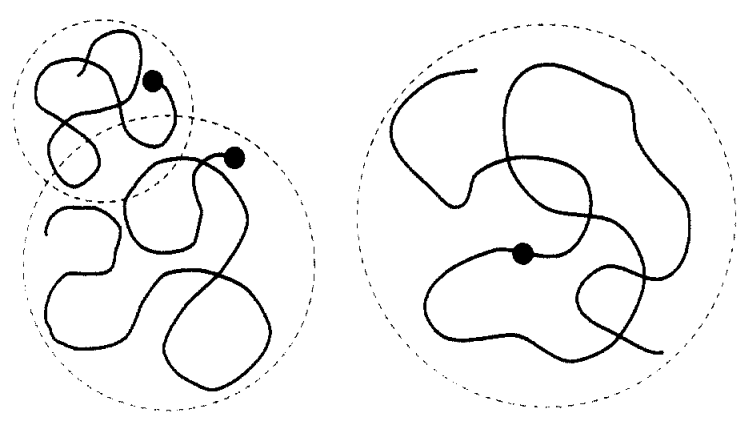
Although the intrinsic reactivity of the free radicals does not change much, the probability that two radicals will approach and annihilate each other will be rather small since the kinetics of the termination will be dominated by entanglement and (chain-end) diffusion. In fact, the reaction rate between two polymers of very different length will be entirely determined by the shorter chain and the rate of termination is given by a power law5:
kt ∼N-α φ-β
where φ is the volume fraction of polymer and N is the average chain length.
The consequence on termination reactions is dramatic; since
N is large, the net rate of termination in the autoacceleration
regime will dramatically decrease, whereas the reactivity of the
monomers will not change much due to the small size of the monomers.
In fact, the concentration of active radicals will rise to a much higher level, and consequently, the
consumption of monomer will increase proportionately.
Another important consequence is, that the addition of a polymer,
like a rubber toughener, will shift the Trommsdorff effect to lower
polymer concentrations, φ.
O'Shaughnessy and Yu5 found that both the slope of conversion and width of
the autoaccleration region are independent of initiator species and concentration.
References
- R. Norrish and R. Smith, Nature 150, 336 (1951)
- E. Trommsdorff, H Koehle, and P Lagally, Makromol. Chem. 1, 169 (1948)
- G.V. Schulz and G. Harborth, Macromol. Chem. 1, 106 (1947)
- P. J. Flory, Principles of Polymer Chemistry, New York 1953
- B. O'Shaughnessy, J. Yu, Macromolecules 27, 5067 (1994)